Vaccines platforms and COVID-19: what you need to know
- PMID: 35965345
- PMCID: PMC9537331
- DOI: 10.1186/s40794-022-00176-4
Vaccines platforms and COVID-19: what you need to know
Abstract
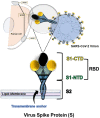
Background: The novel SARS-CoV-2, responsible for the COVID-19 pandemic, is the third zoonotic coronavirus since the beginning of the 21 first century, and it has taken more than 6 million human lives because of the lack of immunity causing global economic losses. Consequently, developing a vaccine against the virus represents the fastest way to finish the threat and regain some "normality."
Objective: Here, we provide information about the main features of the most important vaccine platforms, some of them already approved, to clear common doubts fostered by widespread misinformation and to reassure the public of the safety of the vaccination process and the different alternatives presented.
Methods: Articles published in open access databases until January 2022 were identified using the search terms "SARS-CoV-2," "COVID-19," "Coronavirus," "COVID-19 Vaccines," "Pandemic," COVID-19, and LMICs or their combinations.
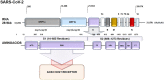
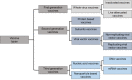
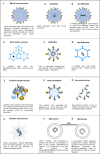
Discussion: Traditional first-generation vaccine platforms, such as whole virus vaccines (live attenuated and inactivated virus vaccines), as well as second-generation vaccines, like protein-based vaccines (subunit and viral vector vaccines), and third-generation vaccines, such as nanoparticle and genetic vaccines (mRNA vaccines), are described.
Conclusions: SARS-CoV-2 sequence information obtained in a record time provided the basis for the fast development of a COVID-19 vaccine. The adaptability characteristic of the new generation of vaccines is changing our capability to react to emerging threats to future pandemics. Nevertheless, the slow and unfair distribution of vaccines to low- and middle-income countries and the spread of misinformation are a menace to global health since the unvaccinated will increase the chances for resurgences and the surge of new variants that can escape the current vaccines.
Keywords: Advantages and disadvantages, first–second- and third-generation vaccines; COVID-19; SARS-CoV-2; Vaccine types; Vaccines platforms; mRNA vaccines.
© 2022. The Author(s).
Conflict of interest statement
"The authors declare that they have no competing interests".
Figures

References
-
- World Health Organization. COVID-19 vaccine development, Coronavirus Update 37. World Health Organization. 2020 p. 28. Available from: https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/... cited 10 May 2021.
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/ cited 2021 Apr 13.
Publication types
Grants and funding
- Grant number 2020000119: "Strengthening of installed capacities of CTeI of the Biomedical/minciencias
- Toxicological/minciencias
- Environmental Sciences Research Group to address problems associated with high biological risks agents for human health in the Bolivar Department/minciencias
- " by SGR./minciencias
LinkOut - more resources
Full Text Sources
Miscellaneous

