Systematic profiling of the chicken gut microbiome reveals dietary supplementation with antibiotics alters expression of multiple microbial pathways with minimal impact on community structure
- PMID: 35965349
- PMCID: PMC9377095
- DOI: 10.1186/s40168-022-01319-7
Systematic profiling of the chicken gut microbiome reveals dietary supplementation with antibiotics alters expression of multiple microbial pathways with minimal impact on community structure
Abstract
Background: The emergence of antimicrobial resistance is a major threat to global health and has placed pressure on the livestock industry to eliminate the use of antibiotic growth promotants (AGPs) as feed additives. To mitigate their removal, efficacious alternatives are required. AGPs are thought to operate through modulating the gut microbiome to limit opportunities for colonization by pathogens, increase nutrient utilization, and reduce inflammation. However, little is known concerning the underlying mechanisms. Previous studies investigating the effects of AGPs on the poultry gut microbiome have largely focused on 16S rDNA surveys based on a single gastrointestinal (GI) site, diet, and/or timepoint, resulting in an inconsistent view of their impact on community composition.
Methods: In this study, we perform a systematic investigation of both the composition and function of the chicken gut microbiome, in response to AGPs. Birds were raised under two different diets and AGP treatments, and 16S rDNA surveys applied to six GI sites sampled at three key timepoints of the poultry life cycle. Functional investigations were performed through metatranscriptomics analyses and metabolomics.
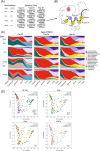
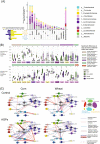
Results: Our study reveals a more nuanced view of the impact of AGPs, dependent on age of bird, diet, and intestinal site sampled. Although AGPs have a limited impact on taxonomic abundances, they do appear to redefine influential taxa that may promote the exclusion of other taxa. Microbiome expression profiles further reveal a complex landscape in both the expression and taxonomic representation of multiple pathways including cell wall biogenesis, antimicrobial resistance, and several involved in energy, amino acid, and nucleotide metabolism. Many AGP-induced changes in metabolic enzyme expression likely serve to redirect metabolic flux with the potential to regulate bacterial growth or produce metabolites that impact the host.
Conclusions: As alternative feed additives are developed to mimic the action of AGPs, our study highlights the need to ensure such alternatives result in functional changes that are consistent with site-, age-, and diet-associated taxa. The genes and pathways identified in this study are therefore expected to drive future studies, applying tools such as community-based metabolic modeling, focusing on the mechanistic impact of different dietary regimes on the microbiome. Consequently, the data generated in this study will be crucial for the development of next-generation feed additives targeting gut health and poultry production. Video Abstract.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

