DDX5 mRNA-targeting antisense oligonucleotide as a new promising therapeutic in combating castration-resistant prostate cancer
- PMID: 35965411
- PMCID: PMC9931527
- DOI: 10.1016/j.ymthe.2022.08.005
DDX5 mRNA-targeting antisense oligonucleotide as a new promising therapeutic in combating castration-resistant prostate cancer
Erratum in
-
DDX5 mRNA-targeting antisense oligonucleotide as a new promising therapeutic in combating castration-resistant prostate cancer.Mol Ther. 2025 Oct 1;33(10):5282. doi: 10.1016/j.ymthe.2025.08.041. Epub 2025 Sep 11. Mol Ther. 2025. PMID: 40939586 No abstract available.
Abstract
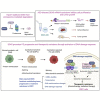
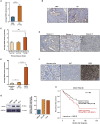
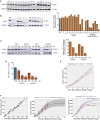
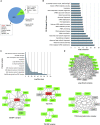
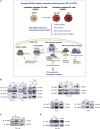
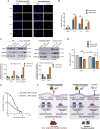
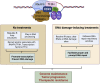
The heat shock protein 27 (Hsp27) has emerged as a principal factor of the castration-resistant prostate cancer (CRPC) progression. Also, an antisense oligonucleotide (ASO) against Hsp27 (OGX-427 or apatorsen) has been assessed in different clinical trials. Here, we illustrate that Hsp27 highly regulates the expression of the human DEAD-box protein 5 (DDX5), and we define DDX5 as a novel therapeutic target for CRPC treatment. DDX5 overexpression is strongly correlated with aggressive tumor features, notably with CRPC. DDX5 downregulation using a specific ASO-based inhibitor that acts on DDX5 mRNAs inhibits cell proliferation in preclinical models, and it particularly restores the treatment sensitivity of CRPC. Interestingly, through the identification and analysis of DDX5 protein interaction networks, we have identified some specific functions of DDX5 in CRPC that could contribute actively to tumor progression and therapeutic resistance. We first present the interactions of DDX5 and the Ku70/80 heterodimer and the transcription factor IIH, thereby uncovering DDX5 roles in different DNA repair pathways. Collectively, our study highlights critical functions of DDX5 contributing to CRPC progression and provides preclinical proof of concept that a combination of ASO-directed DDX5 inhibition with a DNA damage-inducing therapy can serve as a highly potential novel strategy to treat CRPC.
Keywords: DDX5; DNA damage response (DDR); Hsp27; antisense oligonucleotides (ASOs); castration-resistant prostate cancer (CRPC); protein interactions; therapeutic ressistance.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare to have no financial, personal, or professional competing interest and no conflict of interest.
Figures

References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68:394–424. - PubMed
-
- Katsogiannou M., Ziouziou H., Karaki S., Andrieu C., Henry de Villeneuve M., Rocchi P. The hallmarks of castration-resistant prostate cancers. Cancer Treat. Rev. 2015;41:588–597. - PubMed
-
- Rocchi P., Jugpal P., So A., Sinneman S., Ettinger S., Fazli L., Nelson C., Gleave M. Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro. BJU Int. 2006;98:1082–1089. - PubMed
-
- Rocchi P., Beraldi E., Ettinger S., Fazli L., Vessella R.L., Nelson C., Gleave M. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005;65:11083–11093. - PubMed
-
- Rocchi P., So A., Kojima S., Signaevsky M., Beraldi E., Fazli L., Hurtado-Coll A., Yamanaka K., Gleave M. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004;64:6595–6602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

