Uterine perivascular epithelioid tumors (PEComas) with lung metastasis showed good responses to mTOR and VEGFR inhibitors: A case report
- PMID: 35965503
- PMCID: PMC9366196
- DOI: 10.3389/fonc.2022.797275
Uterine perivascular epithelioid tumors (PEComas) with lung metastasis showed good responses to mTOR and VEGFR inhibitors: A case report
Abstract
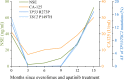
Perivascular epithelioid cell tumors (PEComas) are extremely rare mesenchymal neoplasms for which the uterus is the most common site. The prognosis of malignant PEComa is poor as it is characterized by resistance to classical chemotherapies. Both mTOR inhibitors and VEGFR inhibitors exhibited clinical utility in treating malignant PEComas, but the combination of these two regimens has rarely been reported. In the present case, a uterine PEComa patient developed lung and bone metastases after the failure of chemotherapies and derived benefit from the combination regimen of an mTOR inhibitor (everolimus) and a VEGFR inhibitor (apatinib), achieving a 15-month progression-free survival. Targeted NGS revealed TP53 and TSC2 mutations in the patient's primary uterine tumors and plasma ctDNA at disease progression. Plasma ctDNA clearance was consistent with a radiologic partial response determined by RECIST 1.1 and a reduction of neuron-specific enolase (NSE) and cancer antigen 125 (CA125) levels. Thus, we provided clinical evidence supporting the administration of combined therapy of mTOR and VEGFR inhibitors to metastatic uterine PEComa patients and highlighted the application of serial plasma ctDNA profiling for dynamic disease monitoring.
Keywords: TSC2; apatinib; everolimus; lung metastasis; uterine PEComa.
Copyright © 2022 Sui, Wu, Mei, Pan, Yang, Wu, Ma, Ou and Song.
Conflict of interest statement
Authors EP, PY, TW, YM, and QO are employed by Nanjing Geneseeq Technology Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

