Pro-inflammatory cytokines in cystic glioblastoma: A quantitative study with a comparison with bacterial brain abscesses. With an MRI investigation of displacement and destruction of the brain tissue surrounding a glioblastoma
- PMID: 35965529
- PMCID: PMC9372434
- DOI: 10.3389/fonc.2022.846674
Pro-inflammatory cytokines in cystic glioblastoma: A quantitative study with a comparison with bacterial brain abscesses. With an MRI investigation of displacement and destruction of the brain tissue surrounding a glioblastoma
Abstract
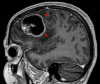
Cystic glioblastomas are aggressive primary brain tumors that may both destroy and displace the surrounding brain tissue as they grow. The mechanisms underlying these tumors' destructive effect could include exposure of brain tissue to tumor-derived cytokines, but quantitative cytokine data are lacking. Here, we provide quantitative data on leukocyte markers and cytokines in the cyst fluid from 21 cystic glioblastomas, which we compare to values in 13 brain abscess pus samples. The concentration of macrophage/microglia markers sCD163 and MCP-1 was higher in glioblastoma cyst fluid than in brain abscess pus; lymphocyte marker sCD25 was similar in cyst fluid and pus, whereas neutrophil marker myeloperoxidase was higher in pus. Median cytokine levels in glioblastoma cyst fluid were high (pg/mL): TNF-α: 32, IL-6: 1064, IL-8: 23585, tissue factor: 28, the chemokine CXCL1: 639. These values were not significantly different from values in pus, pointing to a highly pro-inflammatory glioblastoma environment. In contrast, levels of IFN-γ, IL-1β, IL-2, IL-4, IL-10, IL-12, and IL-13 were higher in pus than in glioblastoma cyst fluid. Based on the quantitative data, we show for the first time that the concentrations of cytokines in glioblastoma cyst fluid correlate with blood leukocyte levels, suggesting an important interaction between glioblastomas and the circulation. Preoperative MRI of the cystic glioblastomas confirmed both destruction and displacement of brain tissue, but none of the cytokine levels correlated with degree of brain tissue displacement or peri-tumoral edema, as could be assessed by MRI. We conclude that cystic glioblastomas are highly pro-inflammatory environments that interact with the circulation and that they both displace and destroy brain tissue. These observations point to the need for neuroprotective strategies in glioblastoma therapy, which could include an anti-inflammatory approach.
Keywords: brain abscess; cyst fluid; cytokine; glioblastoma; inflammation; macrophage; pus; tumor microenvironment.
Copyright © 2022 Hassel, Niehusmann, Halvorsen and Dahlberg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

