Necroptosis-associated long noncoding RNAs can predict prognosis and differentiate between cold and hot tumors in ovarian cancer
- PMID: 35965557
- PMCID: PMC9366220
- DOI: 10.3389/fonc.2022.967207
Necroptosis-associated long noncoding RNAs can predict prognosis and differentiate between cold and hot tumors in ovarian cancer
Abstract
Objective: The mortality rate of ovarian cancer (OC) is the highest among all gynecologic cancers. To predict the prognosis and the efficacy of immunotherapy, we identified new biomarkers.
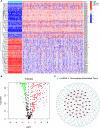
Methods: The Cancer Genome Atlas (TCGA) and the Genotype-Tissue Expression Project (GTEx) databases were used to extract ovarian cancer transcriptomes. By performing the co-expression analysis, we identified necroptosis-associated long noncoding RNAs (lncRNAs). We used the least absolute shrinkage and selection operator (LASSO) to build the risk model. The qRT-PCR assay was conducted to confirm the differential expression of lncRNAs in the ovarian cancer cell line SK-OV-3. Gene Set Enrichment Analysis, Kaplan-Meier analysis, and the nomogram were used to determine the lncRNAs model. Additionally, the risk model was estimated to evaluate the efficacy of immunotherapy and chemotherapy. We classified necroptosis-associated IncRNAs into two clusters to distinguish between cold and hot tumors.
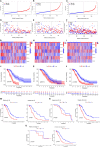
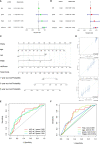
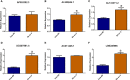
Results: The model was constructed using six necroptosis-associated lncRNAs. The calibration plots from the model showed good consistency with the prognostic predictions. The overall survival of one, three, and five-year areas under the ROC curve (AUC) was 0.691, 0.678, and 0.691, respectively. There were significant differences in the IC50 between the risk groups, which could serve as a guide to systemic treatment. The results of the qRT-PCR assay showed that AL928654.1, AL133371.2, AC007991.4, and LINC00996 were significantly higher in the SK-OV-3 cell line than in the Iose-80 cell line (P < 0.05). The clusters could be applied to differentiate between cold and hot tumors more accurately and assist in accurate mediation. Cluster 2 was more vulnerable to immunotherapies and was identified as the hot tumor.
Conclusion: Necroptosis-associated lncRNAs are reliable predictors of prognosis and can provide a treatment strategy by screening for hot tumors.
Keywords: TCGA; immunotherapy; long noncoding RNAs; necroptosis; ovarian cancer.
Copyright © 2022 He, Fang, Hu, Chen, Shen, Chen, Mu, Li, Zhang, Yong-lin and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

