Return to ovulation after Sayana Press is injected every 4 months for one year: Empirical and pharmacokinetic/pharmacodynamic modeling results
- PMID: 35965654
- PMCID: PMC9372597
- DOI: 10.1016/j.conx.2022.100080
Return to ovulation after Sayana Press is injected every 4 months for one year: Empirical and pharmacokinetic/pharmacodynamic modeling results
Abstract
Objective: To characterize return to ovulation after injecting Sayana Press (104 mg/0.65 mL medroxyprogesterone acetate [MPA] in the Uniject device) every 4 months for 1 year of treatment.
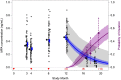
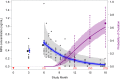
Study design: We followed a subset of women for return to ovulation in a trial that demonstrated Sayana Press remains highly effective when the subcutaneous reinjection interval is extended from 3 to 4 months. We measured serum progesterone in weeks 38 to 42 and 46 to 50 after a final (third) injection and used a concentration ≥4.7 ng/mL as a surrogate for ovulation. We also performed pharmacokinetic and pharmacodynamic modeling to predict differences in MPA accumulation and return to ovulation had - contrary to fact - injections been given every 3 months.
Results: Ten of 19 women (53%; 95% confidence interval: 29-76) ovulated within 50 weeks of their last injection. We predicted that typical 12-month trough MPA concentrations are 34% lower (0.46 vs 0.69 ng/mL) and the median time from last dose to ovulation is 1.1 months shorter (13.1 vs 14.2 months) when injections are given every four months for 1 year.
Conclusion: Extending the Sayana Press reinjection interval from 3 to 4 months leads to less drug accumulation, without a noticeable loss in efficacy. Although the Sayana Press patient leaflet specifies that over 80% of women desiring pregnancy will conceive within a year of stopping the method (independent of treatment duration), our empirical and modeling results indicate women should anticipate waiting a year or more for fertility to return after repeat dosing, with a somewhat shorter delay were the reinjection interval extended to four months.
Implications: Providers should counsel women regarding the distinct possibility that return to fertility will take a year or longer following repeat use of Sayana Press. Extending the dosing interval from 3 to 4 months would result in approximately a 1-month shorter delay, without any appreciable reduction in contraceptive efficacy.
Keywords: Depo-subQ provera; Medroxyprogesterone acetate; Pharmacodynamics; Pharmacokinetics.
© 2022 The Authors. Published by Elsevier Inc.
Figures
Similar articles
-
Ovulation suppression following subcutaneous administration of depot medroxyprogesterone acetate.Contracept X. 2022 Feb 23;4:100073. doi: 10.1016/j.conx.2022.100073. eCollection 2022. Contracept X. 2022. PMID: 35281554 Free PMC article.
-
Lunelle monthly contraceptive injection (medroxyprogesterone acetate and estradiol cypionate injectable suspension): assessment of return of ovulation after three monthly injections in surgically sterile women.Contraception. 1999 Oct;60(4):189-200. doi: 10.1016/s0010-7824(99)00081-5. Contraception. 1999. PMID: 10640165
-
Acceptability of the contraceptive Sayana® Press when injected every four months: Results from a twelve-month trial in Brazil, Chile and the Dominican Republic.Contraception. 2022 Sep;113:95-100. doi: 10.1016/j.contraception.2022.04.007. Epub 2022 Apr 26. Contraception. 2022. PMID: 35483431 Clinical Trial.
-
Pharmacokinetics of depot medroxyprogesterone acetate contraception.J Reprod Med. 1996 May;41(5 Suppl):381-90. J Reprod Med. 1996. PMID: 8725700 Review.
-
Home-based administration of Sayana® Press: review and assessment of needs in low-resource settings.Contraception. 2014 May;89(5):344-51. doi: 10.1016/j.contraception.2014.03.003. Epub 2014 Mar 12. Contraception. 2014. PMID: 24813924 Review.
Cited by
-
Assessing the effect of concerns about contraceptive-induced fertility impairment on hormonal contraceptive use by parity and residence: evidence from PMA Ethiopia 2020 cross-sectional survey.BMJ Open. 2024 Aug 13;14(8):e077192. doi: 10.1136/bmjopen-2023-077192. BMJ Open. 2024. PMID: 39142681 Free PMC article.
-
Potential biomarkers to predict return to fertility after discontinuation of female contraceptives-looking to the future.Front Reprod Health. 2023 Aug 22;5:1210083. doi: 10.3389/frph.2023.1210083. eCollection 2023. Front Reprod Health. 2023. PMID: 37674657 Free PMC article. Review.
References
-
- Pharmacia and Upjohn, Division of Pfizer Inc.; New York, NY: 2020. DEPO-subQ provera [U.S. physician prescribing information]https://www.pfizer.com/products/product-detail/depo_subq_provera_104 (accessed on February 26, 2022)
-
- Jain J., Jakimiuk A.J., Bode F.R., Ross D., Kaunitz A.M. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269–275. - PubMed
-
- Burke H., Chen M., Buluzi M., Fuchs R., Wevill S., Venkatasubramanian L., et al. Effect of self-administration versus provider-administered injection of subcutaneous depot medroxyprogesterone acetate on continuation rates in Malawi: a randomized controlled trial. Lancet Glob Health. 2018;6:e568–e578. - PubMed
-
- Medicines and healthcare products regulatory agency (MHRA), public assessment report, mutual recognition procedure, Sayana Press 104 mg/0.65 mL Suspension for Injection (last updated August 2015). https://products.mhra.gov.uk (accessed on February 13, 2022).
LinkOut - more resources
Full Text Sources

