Incidence of community acquired lower respiratory tract disease in Bristol, UK during the COVID-19 pandemic: A prospective cohort study
- PMID: 35965672
- PMCID: PMC9359590
- DOI: 10.1016/j.lanepe.2022.100473
Incidence of community acquired lower respiratory tract disease in Bristol, UK during the COVID-19 pandemic: A prospective cohort study
Abstract
Background: The emergence of COVID-19 and public health measures implemented to reduce SARS-CoV-2 infections have both affected acute lower respiratory tract disease (aLRTD) epidemiology and incidence trends. The severity of COVID-19 and non-SARS-CoV-2 aLRTD during this period have not been compared in detail.
Methods: We conducted a prospective cohort study of adults age ≥18 years admitted to either of two acute care hospitals in Bristol, UK, from August 2020 to November 2021. Patients were included if they presented with signs or symptoms of aLRTD (e.g., cough, pleurisy), or a clinical or radiological aLRTD diagnosis.
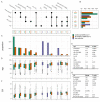
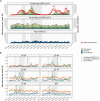
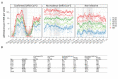
Findings: 12,557 adult aLRTD hospitalisations occurred: 10,087 were associated with infection (pneumonia or non-pneumonic lower respiratory tract infection [NP-LRTI]), 2161 with no infective cause, with 306 providing a minimal surveillance dataset. Confirmed SARS-CoV-2 infection accounted for 32% (3178/10,087) of respiratory infections. Annual incidences of overall, COVID-19, and non- SARS-CoV-2 pneumonia were 714.1, 264.2, and 449.9, and NP-LRTI were 346.2, 43.8, and 302.4 per 100,000 adults, respectively. Weekly incidence trends in COVID-19 aLRTD showed large surges (median 6.5 [IQR 0.7-10.2] admissions per 100,000 adults per week), while other infective aLRTD events were more stable (median 14.3 [IQR 12.8-16.4] admissions per 100,000 adults per week) as were non-infective aLRTD events (median 4.4 [IQR 3.5-5.5] admissions per 100,000 adults per week).
Interpretation: While COVID-19 disease was a large component of total aLRTD during this pandemic period, non- SARS-CoV-2 infection still caused the majority of respiratory infection hospitalisations. COVID-19 disease showed significant temporal fluctuations in frequency, which were less apparent in non-SARS-CoV-2 infection. Despite public health interventions to reduce respiratory infection, disease incidence remains high.
Funding: AvonCAP is an investigator-led project funded under a collaborative agreement by Pfizer.
Keywords: CAP, community acquired pneumonia; COPD, chronic obstructive pulmonary disease; COVID-19; COVID-19, Coronavirus disease 2019; CRDE, chronic respiratory disease exacerbation; Cardiac failure; HF, heart failure; Lower respiratory tract infection; NP-LRTI, non-pneumonic lower respiratory tract infection; Pneumonia; SARS-CoV-2; aLRTD, acute lower respiratory tract disease.
© 2022 Published by Elsevier Ltd.
Conflict of interest statement
CH is Principal Investigator of the Avon CAP study which is an investigator-led University of Bristol study funded by Pfizer and has previously received support from the NIHR in an Academic Clinical Fellowship. JO is a Co-Investigator on the Avon CAP Study. LD is further supported by UKRI through the JUNIPER consortium (grant number MR/V038613/1), MRC (grant number MC/PC/19067), EPSRC (EP/V051555/1 and The Alan Turing Institute, grant EP/N510129/1). AF is a member of the Joint Committee on Vaccination and Immunization (JCVI) and chair of the World Health Organization European Technical Advisory Group of Experts on Immunization (ETAGE) committee. In addition to receiving funding from Pfizer as Chief Investigator of this study, he leads another project investigating transmission of respiratory bacteria in families jointly funded by Pfizer and the Gates Foundation and is an investigator in trials of COVID19 vaccines including ChAdOx1nCOV-19, Janssen and Valneva vaccines. EB, JS, JC, SG, RH, SV, AV, JM, GE, and BG are employees of Pfizer and own Pfizer stock. The other authors have no relevant conflicts of interest to declare. The AvonCAP study is a University of Bristol sponsored study which is investigator-led, and funded under a collaborative agreement by Pfizer Inc.
Figures
References
-
- Pick H, Daniel P, Rodrigo C, et al. Pneumococcal serotype trends, surveillance and risk factors in UK adult pneumonia, 2013–18. Thorax. 2020;75(1):38–49. - PubMed
-
- Elston JW, Santaniello-Newton A, Meigh JA, et al. Increasing incidence of invasive pneumococcal disease and pneumonia despite improved vaccination uptake: surveillance in Hull and East Yorkshire, UK, 2002–2009. Epidemiol Infect. 2012;140(7):1252–1266. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

