Radical C(sp3)-H functionalization and cross-coupling reactions
- PMID: 35965690
- PMCID: PMC9364982
- DOI: 10.1038/s41570-022-00388-4
Radical C(sp3)-H functionalization and cross-coupling reactions
Abstract
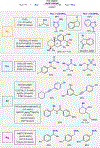
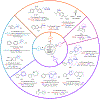
C─H functionalization reactions are playing an increasing role in the preparation and modification of complex organic molecules, including pharmaceuticals, agrochemicals, and polymer precursors. Radical C─H functionalization reactions, initiated by hydrogen-atom transfer (HAT) and proceeding via open-shell radical intermediates, have been expanding rapidly in recent years. These methods introduce strategic opportunities to functionalize C(sp3)─H bonds. Examples include synthetically useful advances in radical-chain reactivity and biomimetic radical-rebound reactions. A growing number of reactions, however, proceed via "radical relay" whereby HAT generates a diffusible radical that is functionalized by a separate reagent or catalyst. The latter methods provide the basis for versatile C─H cross-coupling methods with diverse partners. In the present review, highlights of recent radical-chain and radical-rebound methods provide context for a survey of emerging radical-relay methods, which greatly expand the scope and utility of intermolecular C(sp3)─H functionalization and cross coupling.
Figures









References
-
- Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J & Baudoin O Functionalization of organic molecules by transition-metal-catalyzed C(sp3)─H activation. Chem. Eur. J 16, 2654–2672 (2010). - PubMed
-
- Gupta A, Kumar J, Rahaman A, Singh AK & Bhadra S Functionalization of C(sp3)─H bonds adjacent to heterocycles catalyzed by earth abundant transition metals. Tetrahedron 98, 132415 (2021).
Grants and funding
LinkOut - more resources
Full Text Sources

