Interleukin-35 attenuates blood-brain barrier dysfunction caused by cerebral ischemia-reperfusion injury through inhibiting brain endothelial cell injury
- PMID: 35965799
- PMCID: PMC9372693
- DOI: 10.21037/atm-22-2770
Interleukin-35 attenuates blood-brain barrier dysfunction caused by cerebral ischemia-reperfusion injury through inhibiting brain endothelial cell injury
Abstract
Background: Interleukin-35 (IL-35), an anti-inflammatory and antioxidant cytokine, plays a potent immunosuppressive role in various diseases. However, the effects of IL-35 on blood-brain barrier (BBB) dysfunction in ischemic stroke are not well characterized.
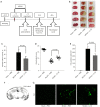
Methods: A total of 150 male C57BL/6 mice (aged 6-8 weeks and weighing 20-25 g) were used in this study. The protective effects of IL-35 against BBB dysfunction were examined using a mouse model of middle cerebral artery occlusion (MCAO) and an in vitro model of oxygen-glucose deprivation/reoxygenation (OGD/R) injury in mouse brain endothelial cells (bEnd.3).
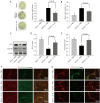
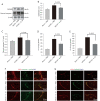
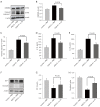
Results: Intracerebroventricular administration of IL-35 (10 µg/g) was found to reduce cerebral edema and Evans blue (EB) leakage, and increase the expression of tight junction (TJ) proteins, thereby attenuating MCAO-induced neurological deficit in mice. Moreover, IL-35 (20 ng/mL) treatment upregulated the expression of TJ proteins in OGD/R-induced bEnd.3 cells. IL-35 also markedly suppressed the expression of caspase-1, IL-1β, and gasdermin D (GSDMD) in vivo and in vitro. In addition, IL-35 decreased the generation of reactive oxygen species (ROS) and inhibited the expression of thioredoxin-interacting protein (TXNIP) in OGD/R-induced bEnd.3 cells.
Conclusions: These results indicated that IL-35 exerts a protective effect on the BBB by targeting the ROS/TXNIP/caspase-1 pathway in cerebral ischemia-reperfusion (I/R) injury.
Keywords: Ischemic stroke; blood-brain barrier (BBB); caspase-1; interleukin-35 (IL-35).
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2770/coif). The authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
