Rhamnocitrin decreases fibrosis of ovarian granulosa cells by regulating the activation of the PPARγ/NF-κB/TGF-β1/Smad2/3 signaling pathway mediated by Wisp2
- PMID: 35965823
- PMCID: PMC9372664
- DOI: 10.21037/atm-22-2496
Rhamnocitrin decreases fibrosis of ovarian granulosa cells by regulating the activation of the PPARγ/NF-κB/TGF-β1/Smad2/3 signaling pathway mediated by Wisp2
Abstract
Background: Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility in women. Rhamnocitrin (Rha) has anti-inflammatory and antioxidant actions. The WNT1-inducible-signaling pathway protein 2 (Wisp2) and nuclear factor (NF)-κB are involved in fibrosis in many diseases. We aimed to elucidate the role of Rha in fibrosis of PCOS and the underlying mechanisms.
Methods: Dehydroepiandrosterone (DHEA)-incubated ovarian granulosa KGN cells were treated by Rha. Cell proliferation was detected with cell counting kit-8 (CCK-8) and 5-ethynyul-2'-deoxyuridine (EdU) staining. The levels of Wisp2 and transforming growth factor-β1 (TGF-β1) in supernatant were measured by enzyme-linked immunosorbent assay (ELISA). We observed α-smooth muscle actin (α-SMA) protein by immunofluorescence (IF). The levels of fibrosis factors were determined using Western blot. We observed p65 nuclear translocation with confocal microscopy. We used Wisp2 overexpression and knockdown in cells treated with DHEA or Rha to validate Wisp2 function. Interaction between Wisp2 and NF-κB, as well as Wisp2 and PPARγ, were assessed by co-immunoprecipitation assay, luciferase reporter assay and chromatin immunoprecipitation (ChIP).
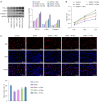
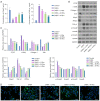
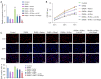
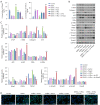
Results: The results showed that Rha elevated the reduced proliferation of DHEA-treated cells. In addition, Rha reversed the decreased Wisp2 and the increased TGF-β1 in supernatant. The proteins CTGF, α-SMA, Collagen I, TGF-β1, p-Smad2, and p-Smad3 were up-regulated while Wisp2, Sirt1, and PPARγ were down-regulated by DHEA treatment, which were reversed by Rha. Meanwhile, DHEA up-regulated p-IKBa and p-p65 and promoted p65 nuclear translocation, which were inhibited by Rha. These effects of Rha were antagonized by Wisp2 knockdown and were mimicked by Wisp2 overexpression. We confirmed the protein interaction between Wisp2 and NF-κB, along with Wisp2 and PPARγ.
Conclusions: Wisp2-mediated PPARγ/NF-κB/TGF-β1/Smad2/3 signaling contributes to Rha-improved ovarian granulosa cells fibrosis, suggesting Rha as a novel agent for the treatment of PCOS.
Keywords: PPARγ/nuclear factor κB (NF-κB)/TGF-β1/Smad2/3 signaling; Rhamnocitrin (Rha); WNT1-inducible-signaling pathway protein 2 (Wisp2); fibrosis; polycystic ovary syndrome (PCOS).
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2496/coif). CH is from Guangxi Xianzhu Traditional Chinese Medicine Technology Co. Ltd. The other authors have no conflicts of interest to declare.
Figures
Similar articles
-
Anti-renal fibrosis effect of asperulosidic acid via TGF-β1/smad2/smad3 and NF-κB signaling pathways in a rat model of unilateral ureteral obstruction.Phytomedicine. 2019 Feb;53:274-285. doi: 10.1016/j.phymed.2018.09.009. Epub 2018 Sep 5. Phytomedicine. 2019. PMID: 30668407
-
[Chrysin inhibited epithelial-mesenchymal transition of type Ⅱ alveolar epithelial cell by regulating NF-κB/Twist 1 signaling pathway].Zhongguo Zhong Yao Za Zhi. 2021 Jan;46(1):146-154. doi: 10.19540/j.cnki.cjcmm.20200810.402. Zhongguo Zhong Yao Za Zhi. 2021. PMID: 33645064 Chinese.
-
Sirtuin 6 inhibits myofibroblast differentiation via inactivating transforming growth factor-β1/Smad2 and nuclear factor-κB signaling pathways in human fetal lung fibroblasts.J Cell Biochem. 2019 Jan;120(1):93-104. doi: 10.1002/jcb.27128. Epub 2018 Sep 19. J Cell Biochem. 2019. PMID: 30230565
-
Liuweiwuling tablets attenuate BDL-induced hepatic fibrosis via modulation of TGF-β/Smad and NF-κB signaling pathways.J Ethnopharmacol. 2018 Jan 10;210:232-241. doi: 10.1016/j.jep.2017.08.029. Epub 2017 Aug 31. J Ethnopharmacol. 2018. PMID: 28864168
-
CCN5/WISP2 and metabolic diseases.J Cell Commun Signal. 2018 Mar;12(1):309-318. doi: 10.1007/s12079-017-0437-z. Epub 2017 Dec 15. J Cell Commun Signal. 2018. PMID: 29247377 Free PMC article. Review.
Cited by
-
Extracellular matrix dysregulation in PCOS: pathogenesis, therapeutic strategies, and innovative technologies.J Biol Eng. 2025 Jul 5;19(1):61. doi: 10.1186/s13036-025-00533-9. J Biol Eng. 2025. PMID: 40618169 Free PMC article. Review.
-
Signaling pathways and targeted therapeutic strategies for polycystic ovary syndrome.Front Endocrinol (Lausanne). 2023 Oct 19;14:1191759. doi: 10.3389/fendo.2023.1191759. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37929034 Free PMC article. Review.
-
Alternative treatment of polycystic ovary syndrome: pre-clinical and clinical basis for using plant-based drugs.Front Endocrinol (Lausanne). 2024 Jan 11;14:1294406. doi: 10.3389/fendo.2023.1294406. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38725974 Free PMC article. Review.
-
Insights on the NF-κB system in polycystic ovary syndrome, attractive therapeutic targets.Mol Cell Biochem. 2024 Mar;479(3):467-486. doi: 10.1007/s11010-023-04736-w. Epub 2023 Apr 25. Mol Cell Biochem. 2024. PMID: 37097332 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
