Effect of intravenous lidocaine on chronic postoperative pain in patients undergoing breast cancer surgery: a prospective, double-blind, randomized, placebo-controlled clinical trial
- PMID: 35965825
- PMCID: PMC9372649
- DOI: 10.21037/atm-22-3522
Effect of intravenous lidocaine on chronic postoperative pain in patients undergoing breast cancer surgery: a prospective, double-blind, randomized, placebo-controlled clinical trial
Abstract
Background: Chronic postoperative pain (CPSP) is one of the common complications of breast cancer patients, which can seriously affect the quality of life and long-term prognosis of patients. The purpose of this study was to investigate whether perioperative intravenous lidocaine infusion could reduce the incidence of CPSP in patients with breast cancer.
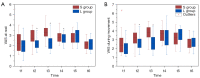
Methods: Female patients undergoing radical breast cancer surgery were randomly assigned to the 2% lidocaine group (L) and the control group (S). group L received an intravenous infusion of 1.5 mg/kg lidocaine 10 minutes prior to induction, followed by a continuous infusion of 2 mg/kg/h until the end of surgery. The control group received an equal amount of saline. The primary outcome was the incidence of CPSP at 3 months. Secondary outcomes included VAS pain scores and frequency of remedial analgesia within 24 hours postoperatively; incidence of CPSP at 1 and 6 months; and scores on the Brief Pain Inventory (BPI), Simplified McGill Pain Questionnaire (SF-MPQ), and Neuropathic Pain Score (DN-4) at 1, 3, and 6 months postoperatively.
Results: Eighty-two patients participated in this study. A total of 78 patients completed the 3-month postoperative follow-up (39 in group S and 39 in group L). At 3 months, the incidence of CPSP was significantly lower in the L group than in the S group (33.3% in the S group and 12.8% in the L group, P=0.032). Pain scores at rest and during exercise were significantly lower in the L group than in the S group at different time points (P≤0.001 and P<0.001). The need for remedial analgesia at 24 hours postoperatively also differed significantly between the two groups (P=0.036). At 6 months, the incidence of CPSP was also lower in the L group than in the S group (29.7% in the S group and 10.5% in the L group, P=0.038). The differences in SF-MPQ scores were statistically significant at both 3 and 6 months postoperatively (P=0.022, P=0.037).
Conclusions: Intravenous infusion of lidocaine reduces the incidence of CPSP in breast cancer patients at 3 and 6 months and is effective in relieving acute postoperative pain.
Trial registration: Chinese Clinical Trial Registry ChiCTR2100050445.
Keywords: Lidocaine; breast cancer; chronic postoperative pain (CPSP).
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3522/coif). The authors have no conflicts of interest to declare.
Figures

References
LinkOut - more resources
Full Text Sources
