Influence of the Synthesis and Crystallization Processes on the Cation Distribution in a Series of Multivariate Rare-Earth Metal-Organic Frameworks and Their Magnetic Characterization
- PMID: 35965890
- PMCID: PMC9367679
- DOI: 10.1021/acs.chemmater.2c01481
Influence of the Synthesis and Crystallization Processes on the Cation Distribution in a Series of Multivariate Rare-Earth Metal-Organic Frameworks and Their Magnetic Characterization
Abstract
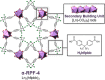
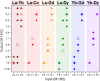
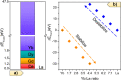
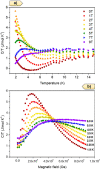
The incorporation of multiple metal atoms in multivariate metal-organic frameworks is typically carried out through a one-pot synthesis procedure that involves the simultaneous reaction of the selected elements with the organic linkers. In order to attain control over the distribution of the elements and to be able to produce materials with controllable metal combinations, it is required to understand the synthetic and crystallization processes. In this work, we have completed a study with the RPF-4 MOF family, which is made of various rare-earth elements, to investigate and determine how the different initial combinations of metal cations result in different atomic distributions in the obtained materials. Thus, we have found that for equimolar combinations involving lanthanum and another rare-earth element, such as ytterbium, gadolinium, or dysprosium, a compositional segregation takes place in the products, resulting in crystals with different compositions. On the contrary, binary combinations of ytterbium, gadolinium, erbium, and dysprosium result in homogeneous distributions. This dissimilar behavior is ascribed to differences in the crystallization pathways through which the MOF is formed. Along with the synthetic and crystallization study and considering the structural features of this MOF family, we also disclose here a comprehensive characterization of the magnetic properties of the compounds and the heat capacity behavior under different external magnetic fields.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Magnetocaloric Properties in Rare-Earth-Based Metal-Organic Frameworks: Influence of Magnetic Density and Hydrostatic Pressure.Inorg Chem. 2023 Dec 4;62(48):19741-19748. doi: 10.1021/acs.inorgchem.3c03138. Epub 2023 Nov 21. Inorg Chem. 2023. PMID: 38044828
-
Stepwise Synthesis of Metal-Organic Frameworks.Acc Chem Res. 2017 Apr 18;50(4):857-865. doi: 10.1021/acs.accounts.6b00457. Epub 2017 Mar 28. Acc Chem Res. 2017. PMID: 28350434
-
Site Isolation in Metal-Organic Frameworks Enables Novel Transition Metal Catalysis.Acc Chem Res. 2018 Sep 18;51(9):2129-2138. doi: 10.1021/acs.accounts.8b00297. Epub 2018 Aug 21. Acc Chem Res. 2018. PMID: 30129753
-
Mixed-metal metal-organic frameworks.Chem Soc Rev. 2019 May 7;48(9):2535-2565. doi: 10.1039/c8cs00337h. Chem Soc Rev. 2019. PMID: 30989162 Review.
-
Expanding the Rare-Earth Metal BINOLate Catalytic Multitool beyond Enantioselective Organic Synthesis.Acc Chem Res. 2021 Jun 1;54(11):2637-2648. doi: 10.1021/acs.accounts.1c00148. Epub 2021 May 20. Acc Chem Res. 2021. PMID: 34014657 Review.
References
-
- Lerma-Berlanga B.; Ganivet R. C.; Tatay N.; Peng S.; Albero Y.; Fabelo J.; González-Platas O.; García J.; Padial M.; Martí-Gastaldo C. Effect of Linker Distribution in the Photocatalytic Activity of Multivariate Mesoporous Crystals. J. Am. Chem. Soc. 2021, 143, 1798–1806. 10.1021/jacs.0c09015. - DOI - PubMed
-
- Castillo-Blas C.; Gándara F. Metal-Organic Frameworks Incorporating Multiple Metal Elements. Isr. J. Chem. 2018, 58, 1036–1043. 10.1002/ijch.201800085. - DOI
LinkOut - more resources
Full Text Sources
