Histological Analysis of the Effect of Nanofat Grafting in Scar Rejuvenation
- PMID: 35965912
- PMCID: PMC9364463
- DOI: 10.4103/JCAS.JCAS_106_21
Histological Analysis of the Effect of Nanofat Grafting in Scar Rejuvenation
Abstract
Introduction: The morphology and tissue response to macro- and micro-fat grafting have been widely studied in both clinical and experimental settings; the histological effects of the nanofat graft, however, remain largely unexplored.
Aims: This study was carried out to evaluate the histological changes leading to scar rejuvenation in a fine scar following nanofat grafting.
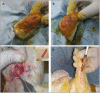
Materials and methods: This was an experimental study carried out on guinea-pig fine-line scar models. Nanofat prepared from abdominal fat of the animal was injected into scar on right legs (NFG) at 1 month whereas left acted as controls (CG). Punch biopsies from all scars were analyzed at 2, 4, and 6 months by Hematoxylin&Eosin, Masson's trichrome, and Picrosirius red stains to evaluate dermal/epidermal regeneration, collagen fiber orientation, pattern of distribution, and amount of mature and immature collagen.
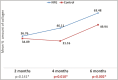
Results: Nine animals were included in the final analysis of the study. On histological analysis, the amount of inflammatory infiltrate, collagen fiber orientation, pattern and total histological score at 2, 4, and 6 months were similar between the groups. There was an increased trend for earlier appearance of organized and mature forms of collagen in the NFG group. The distribution of collagen was similar at 2 months; however, there was a significant increase in collagen distribution in NFG at 4 months (NFG: 46.11±11.6, CG: 31.16±9.9; P = 0.010) and at 6 months (NFG: 63.48± 6.6, CG: 49.9 ±8.8; P = 0.002).
Conclusion: Nanofat grafting is associated with an accelerated and increased production of mature collagen with proper alignment in fine-line scars.
Keywords: Collagen; Tonnard; fat grafting; icrosirius red; scar remodeling.
Copyright: © 2022 Journal of Cutaneous and Aesthetic Surgery.
Conflict of interest statement
There are no conflicts of interest.
Figures


Similar articles
-
Effects of Nanofat in Plastic and Reconstructive Surgery: A Systematic Review.Plast Reconstr Surg. 2024 Sep 1;154(3):451e-464e. doi: 10.1097/PRS.0000000000010905. Epub 2023 Jul 4. Plast Reconstr Surg. 2024. PMID: 37400953
-
Immediate fat and nanofat-enriched fat grafting in breast reduction for scar management.J Plast Surg Hand Surg. 2021 Jun;55(3):173-180. doi: 10.1080/2000656X.2020.1856678. Epub 2020 Dec 14. J Plast Surg Hand Surg. 2021. PMID: 33315503 Clinical Trial.
-
Autologous emulsified fat injection for rejuvenation of scars: A prospective observational study.Indian J Plast Surg. 2018 Jan-Apr;51(1):77-83. doi: 10.4103/ijps.IJPS_86_17. Indian J Plast Surg. 2018. PMID: 29928084 Free PMC article.
-
Nanofat Grafting for Scar Treatment and Skin Quality Improvement.Aesthet Surg J. 2018 Mar 14;38(4):421-428. doi: 10.1093/asj/sjx183. Aesthet Surg J. 2018. PMID: 29365061
-
Nanofat in Plastic Reconstructive, Regenerative, and Aesthetic Surgery: A Review of Advancements in Face-Focused Applications.J Clin Med. 2023 Jun 28;12(13):4351. doi: 10.3390/jcm12134351. J Clin Med. 2023. PMID: 37445386 Free PMC article. Review.
Cited by
-
Nano Fat Therapy and Platelet Rich Plasma Versus Nano Fat Therapy Alone on Burn Scar.Ann Burns Fire Disasters. 2025 Jun 30;38(2):119-123. eCollection 2025 Jun. Ann Burns Fire Disasters. 2025. PMID: 40589711 Free PMC article.
-
Mechanical Fractionation of Adipose Tissue-A Scoping Review of Procedures to Obtain Stromal Vascular Fraction.Bioengineering (Basel). 2023 Oct 9;10(10):1175. doi: 10.3390/bioengineering10101175. Bioengineering (Basel). 2023. PMID: 37892905 Free PMC article.
References
-
- Piccolo NS, Piccolo MS, Piccolo MT. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clin Plast Surg. 2015;42:263–83. - PubMed
-
- Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: Safety and efficacy. Plast Reconstr Surg. 2007;119:775–85; discussion 786-7. - PubMed
-
- Neuber F. Fat transplantation. Bericht über die Verhandlungen der deutschen Gesellschaft für Chirurgie. Zentralbl Chir. 1893;22:66.
-
- Trepsat F. Midface reshaping with micro-fat grafting. Ann Chir Plast Esthet. 2009;54:435–43. - PubMed
LinkOut - more resources
Full Text Sources
