Regulation of NMDA Receptor Signaling at Single Synapses by Human Anti-NMDA Receptor Antibodies
- PMID: 35966009
- PMCID: PMC9371948
- DOI: 10.3389/fnmol.2022.940005
Regulation of NMDA Receptor Signaling at Single Synapses by Human Anti-NMDA Receptor Antibodies
Abstract
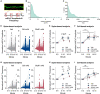
The NMDA receptor (NMDAR) subunit GluN1 is critical for receptor function and plays a pivotal role in synaptic plasticity. Mounting evidence has shown that pathogenic autoantibody targeting of the GluN1 subunit of NMDARs, as in anti-NMDAR encephalitis, leads to altered NMDAR trafficking and synaptic localization. However, the underlying signaling pathways affected by antibodies targeting the NMDAR remain to be fully delineated. It remains unclear whether patient antibodies influence synaptic transmission via direct effects on NMDAR channel function. Here, we show using short-term incubation that GluN1 antibodies derived from patients with anti-NMDAR encephalitis label synapses in mature hippocampal primary neuron culture. Miniature spontaneous calcium transients (mSCaTs) mediated via NMDARs at synaptic spines are not altered in pathogenic GluN1 antibody exposed conditions. Unexpectedly, spine-based and cell-based analyses yielded distinct results. In addition, we show that calcium does not accumulate in neuronal spines following brief exposure to pathogenic GluN1 antibodies. Together, these findings show that pathogenic antibodies targeting NMDARs, under these specific conditions, do not alter synaptic calcium influx following neurotransmitter release. This represents a novel investigation of the molecular effects of anti-NMDAR antibodies associated with autoimmune encephalitis.
Keywords: NMDA receptor; autoimmune encephalitis; calcium signaling; neurotransmission; synaptic.
Copyright © 2022 Dean, Metzbower, Dessain, Blanpied and Benavides.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Adekar S. P., Jones R. M., Elias M. D., Al-Saleem F. H., Root M. J., Simpson L. L., et al. (2008). ‘Hybridoma populations enriched for affinity-matured human IgGs yield high-affinity antibodies specific for botulinum neurotoxins’. J. Immunol. Methods 333 156–166. 10.1016/j.jim.2008.01.015 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

