Upregulation of Netrin-1 in the hippocampus mediates the formation of visceral hypersensitivity induced by maternal separation
- PMID: 35966013
- PMCID: PMC9366914
- DOI: 10.3389/fnmol.2022.908911
Upregulation of Netrin-1 in the hippocampus mediates the formation of visceral hypersensitivity induced by maternal separation
Abstract
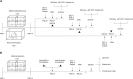
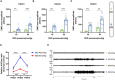
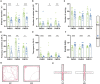
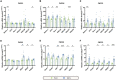
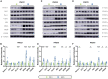
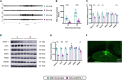
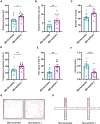
Early adverse life events (EALs), such as maternal separation (MS), can cause visceral hypersensitivity, which is thought to be a key pathophysiological mechanism of irritable bowel syndrome (IBS). Previous studies mainly focused on EALs-induced visceral hypersensitivity in adulthood but did not consider that it may have occurred in the preadult period. We previously found that rats who experienced MS suffered from visceral hypersensitivity starting from the post-weaning period. Moreover, the hippocampus is considered to be critical in regulating the formation of visceral hypersensitivity induced by MS. But the underlying mechanisms throughout different life periods are unclear. In this study, behavioral tests, RNA-seq, lentiviral interference, and molecular biology techniques were applied to investigate the molecular mechanism in the hippocampus underlying MS-induced long-lasting visceral hypersensitivity. It was found that both visceral sensitivity and anxiety-like behaviors were significantly increased in MS rats in post-weaning, prepubertal, and adult periods, especially in the prepubertal period. Subsequently, RNA-seq targeting the hippocampus identified that the expression level of Netrin-1 was significantly increased in all periods, which was further confirmed by quantitative real-time PCR and Western blot. Knocking-down hippocampal Netrin-1 in the post-weaning period by lentivirus interference alleviated visceral hypersensitivity and anxiety-like behaviors of MS rats in the later phase of life. In addition, deleted in colorectal cancer (DCC), instead of neogenin-1(Neo-1) or uncoordinated (UNC5), was proved to be the specific functional receptor of Netrin-1 in regulating visceral hypersensitivity, whose upregulation may result in the most severe symptoms in the prepubertal period. Furthermore, the activation of the Netrin-1/DCC pathway could enhance long-term potentiation (LTP) in the hippocampus, probably via recruitment of the AMPA receptor subunit GluA1, which finally resulted in the formation of visceral hypersensitivity. These novel findings suggest that long-lasting over-expression of Netrin-1 can mediate visceral hypersensitivity and anxiety disorder from the post-weaning period to adulthood by activating DCC/GluA1 pathway in the hippocampus. Moreover, early intervention of Netrin-1 in the post-weaning period could lead to significant symptom relief afterward, which provides evidence that the Netrin-1/DCC/GluA1 signaling pathway may be a potential therapeutic target for the treatment of visceral hypersensitivity in clinics.
Keywords: DCC; GluA1; Netrin-1; hippocampus; maternal separation; visceral hypersensitivity.
Copyright © 2022 Wang, Duan, Zhan, Dong, Zhang, Chen, Sun and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

