Role of nuclear pregnane X receptor in Cu-induced lipid metabolism and xenobiotic responses in largemouth bass (Micropterus salmoides)
- PMID: 35966089
- PMCID: PMC9365941
- DOI: 10.3389/fendo.2022.950985
Role of nuclear pregnane X receptor in Cu-induced lipid metabolism and xenobiotic responses in largemouth bass (Micropterus salmoides)
Abstract
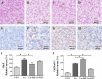
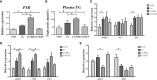
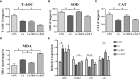
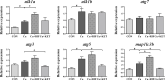
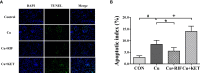
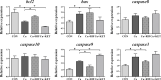
The pregnane X receptor (PXR) is a master xenobiotic-sensing receptor in response to toxic byproducts, as well as a key regulator in intermediary lipid metabolism. Therefore, the present study was conducted to investigate the potential role of PXR in mediating the lipid dysregulation and xenobiotic responses under Cu-induced stress in largemouth bass (Micropterus salmoides). Four groups of largemouth bass (52.66 ± 0.03 g) were treated with control, Cu waterborne (9.44 μmol/L), Cu+RIF (Rifampicin, 100 mg/kg, PXR activator), and Cu+KET (Ketoconazole, 20 mg/kg, PXR inhibitor) for 48 h. Results showed that Cu exposure significantly elevated the plasma stress indicators and triggered antioxidant systems to counteract Cu-induced oxidative stress. Acute Cu exposure caused liver steatosis, as indicated by the significantly higher levels of plasma triglycerides (TG), lipid droplets, and mRNA levels of lipogenesis genes in the liver. Liver injuries were detected, as shown by hepatocyte vacuolization and severe apoptotic signals after Cu exposure. Importantly, Cu exposure significantly stimulated mRNA levels of PXR, suggesting the response of this regulator in the xenobiotic response. The pharmacological intervention of PXR by the agonist and antagonist significantly altered hepatic mRNA levels of PXR, implying that RIF and KET were effective agents of PXR in largemouth bass. Administration of RIF significantly exacerbated liver steatosis, and such alterations were dependent on the regulations on pparγ and cd36 rather than srebp1 signaling, which suggested that PXR-PPARγ might be another pathway for Cu-induced lipid deposition in fish. Whereas, KET administration showed reverse effects on lipid metabolism as indicated by the lower hepatic TG levels, suppressed mRNA levels of pparγ and cd36. Activation of PXR stimulated autophagy and inhibited apoptosis, leading to lower hepatic vacuolization; while inhibition of PXR showed higher apoptotic signals, inhibition of autophagic genes and stimulation of apoptotic genes. Taken together, PXR played a cytoprotective role in Cu-induced hepatotoxicity through regulations on autophagy and apoptosis. Overall, our data has demonstrated for the first time on the dual roles of PXR as a co-regulator in mediating xenobiotic responses and lipid metabolism in fish, which implying the potential of PXR as a therapy target for xenobiotics-induced lipid dysregulation and hepatotoxicity.
Keywords: Cu exposure; PXR; apoptosis; autophagy; lipid metabolism.
Copyright © 2022 Li, Gong, Wang, Yu, Tian, Xia, Li, Zhang and Xie.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
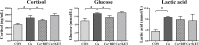
References
-
- Lange A, Corcoran J, Miyagawa S, Iguchi T, Winter MJ, Tyler CR. Development of a common carp (Cyprinus carpio) pregnane X receptor (cPXR) transactivation reporter assay and its activation by azole fungicides and pharmaceutical chemicals. toxicol. In Vitro (2017) 41:114–22. doi: 10.1016/j.tiv.2017.02.023 - DOI - PMC - PubMed
-
- Li H, Xu W, Wu L, Dong B, Jin J, Han D, et al. Differential regulation of endoplasmic reticulum stress-induced autophagy and apoptosis in two strains of gibel carp (Carassius gibelio) exposed to acute waterborne cadmium. Aquat Toxicol (2021) 231:105721. doi: 10.1016/j.aquatox.2020.105721 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

