Impact of Perineuronal Net Removal in the Rat Medial Prefrontal Cortex on Parvalbumin Interneurons After Reinstatement of Cocaine Conditioned Place Preference
- PMID: 35966203
- PMCID: PMC9366391
- DOI: 10.3389/fncel.2022.932391
Impact of Perineuronal Net Removal in the Rat Medial Prefrontal Cortex on Parvalbumin Interneurons After Reinstatement of Cocaine Conditioned Place Preference
Abstract
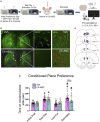
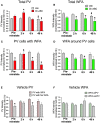
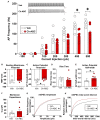
Parvalbumin (PV)-positive cells are GABAergic fast-spiking interneurons that modulate the activity of pyramidal neurons in the medial prefrontal cortex (mPFC) and their output to brain areas associated with learning and memory. The majority of PV cells within the mPFC are surrounded by a specialized extracellular matrix structure called the perineuronal net (PNN). We have shown that removal of PNNs with the enzyme chondroitinase-ABC (Ch-ABC) in the mPFC prevents the consolidation and reconsolidation of cocaine-associated conditioned place preference (CPP) memories. Here we examined the extent to which retrieval of a CPP memory during cocaine-primed reinstatement altered the levels and function of PV neurons and their surrounding PNNs during the reconsolidation period. We further determined the extent to which PNN removal prior to reinstatement altered PV intensity levels and PV cell function. Male Sprague-Dawley rats were trained for cocaine-induced conditioned place preference (CPP) followed by extinction training, microinjection of Ch-ABC in the prelimbic PFC, and cocaine-induced reinstatement. Rats were sacrificed immediately prior to reinstatement or at 2 h, 6 h, or 48 h after reinstatement for immunohistochemistry or 2 h later for electrophysiology. Our findings indicate that PNN removal only partially diminished reinstatement. Cocaine-primed reinstatement produced only minor changes in PNN or PV intensity in vehicle controls. However, after PNN removal, the intensity of remaining PNN-surrounded PV cells was decreased at all times except at 2 h post-reinstatement, at which time cocaine increased PV intensity. Consistent with this, in vehicle controls, PV neurons naturally devoid of PNNs showed a similar pattern to Ch-ABC-treated rats prior to and after cocaine reinstatement, suggesting a protective effect of PNNs on cocaine-induced changes in PV intensity. Using whole-cell patch-clamp, cocaine-primed reinstatement in Ch-ABC-treated rats decreased the number of elicited action potentials but increased excitatory synaptic transmission, which may have been compensatory. These findings suggest that without PNNs, cocaine-induced reinstatement produces rapid changes in PV intensity and PV cell excitability, which may in turn regulate output of the mPFC post-memory retrieval and diminish the maintenance of cocaine memory during reconsolidation.
Keywords: addiction; cocaine; conditioned place preference; memory; parvalbumin; perineuronal nets; prefrontal cortex; substance use disorder.
Copyright © 2022 Gonzalez, Jorgensen, Ramos, Harkness, Aadland, Brown and Sorg.
Conflict of interest statement
JH is the CEO of Rewire Neuro. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Enhanced expression of parvalbumin and perineuronal nets in the medial prefrontal cortex after extended-access cocaine self-administration in rats.Addict Biol. 2023 Nov;28(11):e13334. doi: 10.1111/adb.13334. Addict Biol. 2023. PMID: 37855072
-
Cocaine Exposure Modulates Perineuronal Nets and Synaptic Excitability of Fast-Spiking Interneurons in the Medial Prefrontal Cortex.eNeuro. 2018 Oct 4;5(5):ENEURO.0221-18.2018. doi: 10.1523/ENEURO.0221-18.2018. eCollection 2018 Sep-Oct. eNeuro. 2018. PMID: 30294670 Free PMC article.
-
Cocaine memory reactivation induces functional adaptations within parvalbumin interneurons in the rat medial prefrontal cortex.Addict Biol. 2021 May;26(3):e12947. doi: 10.1111/adb.12947. Epub 2020 Aug 4. Addict Biol. 2021. PMID: 32750200 Free PMC article.
-
The Perineuronal 'Safety' Net? Perineuronal Net Abnormalities in Neurological Disorders.Front Mol Neurosci. 2018 Aug 3;11:270. doi: 10.3389/fnmol.2018.00270. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30123106 Free PMC article. Review.
-
Perineuronal Nets and Metal Cation Concentrations in the Microenvironments of Fast-Spiking, Parvalbumin-Expressing GABAergic Interneurons: Relevance to Neurodevelopment and Neurodevelopmental Disorders.Biomolecules. 2021 Aug 18;11(8):1235. doi: 10.3390/biom11081235. Biomolecules. 2021. PMID: 34439901 Free PMC article. Review.
Cited by
-
Extracellular matrix abnormalities in the hippocampus of subjects with substance use disorder.Transl Psychiatry. 2024 Feb 24;14(1):115. doi: 10.1038/s41398-024-02833-y. Transl Psychiatry. 2024. PMID: 38402197 Free PMC article.
-
Timing of Methamphetamine Exposure during Adolescence Differentially Influences Parvalbumin and Perineuronal Net Immunoreactivity in the Medial Prefrontal Cortex of Female, but Not Male, Rats.Dev Neurosci. 2025;47(1):27-39. doi: 10.1159/000538608. Epub 2024 Mar 28. Dev Neurosci. 2025. PMID: 38547851 Free PMC article.
-
Distinct roles of excitatory and inhibitory neurons in the medial prefrontal cortex in the expression and reconsolidation of methamphetamine-associated memory in male mice.Neuropsychopharmacology. 2024 Nov;49(12):1827-1838. doi: 10.1038/s41386-024-01879-2. Epub 2024 May 10. Neuropsychopharmacology. 2024. PMID: 38730034
-
Enhanced Fear Extinction Through Infralimbic Perineuronal Net Digestion: The Modulatory Role of Adolescent Alcohol Exposure.bioRxiv [Preprint]. 2024 Oct 23:2024.10.23.619810. doi: 10.1101/2024.10.23.619810. bioRxiv. 2024. Update in: Alcohol. 2025 Mar;123:57-67. doi: 10.1016/j.alcohol.2024.12.006. PMID: 39484370 Free PMC article. Updated. Preprint.
-
Perineuronal Nets in the Rat Medial Prefrontal Cortex Alter Hippocampal-Prefrontal Oscillations and Reshape Cocaine Self-Administration Memories.J Neurosci. 2024 Aug 21;44(34):e0468242024. doi: 10.1523/JNEUROSCI.0468-24.2024. J Neurosci. 2024. PMID: 38991791 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

