Lipase-Mediated Mechanoenzymatic Synthesis of Sugar Esters in Dissolved Unconventional and Neat Reaction Systems
- PMID: 35966390
- PMCID: PMC9364441
- DOI: 10.1021/acssuschemeng.2c01727
Lipase-Mediated Mechanoenzymatic Synthesis of Sugar Esters in Dissolved Unconventional and Neat Reaction Systems
Abstract
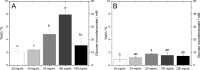
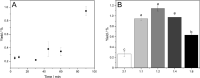
Mechanochemical and biocatalytic approaches in modern research are two major assets to develop greener processes. In the present study, these modular tools of sustainability are pointed toward the production of versatile and daily employed compounds such as surfactants. Toward this aim, glycolipids, a class of nonionic surfactants composed of ubiquitous and primary metabolites such as sugar and fatty acid moieties, represent a promising alternative to petroleum-derived surface-active agents. Therefore, the combination of biocatalysis with mechanochemistry aiming at glycolipid synthesis seemed a logical step that was taken in this study for the first time. The monoacylated model compound glucose-6-O-decanoate was synthesized with the help of a bead mill apparatus using two different unconventional dissolved reaction systems, namely, menthol-based hydrophobic deep eutectic solvents and 2-methyl-2-butanol, thus reaching up to 12% yield in the latter based on the conversion of vinyl decanoate, after only 90 min of reaction. In addition, a neat reaction system using an excess of vinylated fatty ester as an adjuvant allowed a 27 mM/h space-time yield. The overall significant increase in productivities, up to 6 times, compared to standard heating and shaking methods, shows the tremendous potential of mechanoenzymatic synthesis.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Shete A. M.; Wadhawa G.; Banat I. M.; Chopade B. A. Mapping of patents on bioemulsifier and biosurfactant: A review. J. Sci. Ind. Res. 2006, 65, 91–115.
-
- Surfactants Market Research Report: Market Size, Industry Outlook, Market Forecast, Demand Analysis,Market Share, Market Report 2021-2026 [Internet]. Available from: https://www.industryarc.com/Report/15201/surfactants-market.html.
-
- Grüninger J.; Delavault A.; Ochsenreither K. Enzymatic glycolipid surfactant synthesis from renewables. Process Biochem. 2019, 87, 45–54. 10.1016/j.procbio.2019.09.023. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
