Metal Complexes for Therapeutic Applications
- PMID: 35966501
- PMCID: PMC9374106
- DOI: 10.1016/j.trechm.2021.03.006
Metal Complexes for Therapeutic Applications
Abstract
Metal complexes have been widely used for applications in the chemical and physical sciences due to their unique electronic and stereochemical properties. For decades the use of metal complexes for medicinal applications has been postulated and demonstrated. The distinct characteristics of metal complexes, including their molecular geometries (that are not readily accessed by organic molecules), as well as their ligand exchange, redox, catalytic, and photophysical reactions, give these compounds the potential to interact and react with biomolecules in unique ways and by distinct mechanisms of action. Herein, the potential of metal complexes to act as components bioactive therapeutic compounds is discussed.
Keywords: Bioinorganic Chemistry; Chemical Biology; Coordination Compounds; Enzyme Inhibitors; Metals in Medicine.
Conflict of interest statement
Declaration of Interests There are no interests to declare.
Figures


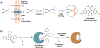
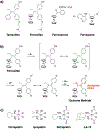
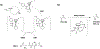
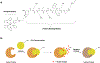
References
-
- Kaufmann SHE (2008) Paul Ehrlich: Founder Of Chemotherapy. Nat. Rev. Drug Discov 7 (5), 373–373. - PubMed
-
- Winau F et al. (2004) Paul Ehrlich — In Search Of The Magic Bullet. Microbes Infect. 6 (8), 786–789. - PubMed
-
- Lloyd NC et al. (2005) The Composition Of Ehrlich’s Salvarsan: Resolution Of A Century-Old Debate. Angew. Chem. Int. Ed 44 (6), 941–944. - PubMed
-
- Rosenberg B et al. (1965) Inhibition Of Cell Division In Escherichia Coli By Electrolysis Products From A Platinum Electrode. Nature 205 (4972), 698–699. - PubMed
-
- Rosenberg B et al. (1969) Platinum Compounds: A New Class Of Potent Antitumour Agents. Nature 222 (5191), 385–386. - PubMed
