Novel Early Pregnancy Multimarker Screening Test for Preeclampsia Risk Prediction
- PMID: 35966513
- PMCID: PMC9363612
- DOI: 10.3389/fcvm.2022.932480
Novel Early Pregnancy Multimarker Screening Test for Preeclampsia Risk Prediction
Abstract
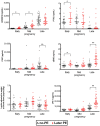
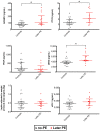
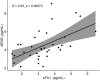
Preeclampsia (PE) is a common pregnancy-linked disease, causing preterm births, complicated deliveries, and health consequences for mothers and offspring. We have previously developed 6PLEX, a multiplex assay that measures PE-related maternal serum biomarkers ADAM12, sENG, leptin, PlGF, sFlt-1, and PTX3 in a single test tube. This study investigated the potential of 6PLEX to develop novel PE prediction models for early pregnancy. We analyzed 132 serum samples drawn at 70-275 gestational days (g days) from 53 pregnant women (PE, n = 22; controls, n = 31). PE prediction models were developed using a machine learning strategy based on the stepwise selection of the most significant models and incorporating parameters with optimal resampling. Alternative models included also placental FLT1 rs4769613 T/C genotypes, a high-confidence risk factor for PE. The best performing PE prediction model using samples collected at 70-98 g days comprised of PTX3, sFlt-1, and ADAM12, the subject's parity and gestational age at sampling (AUC 0.94 [95%CI 0.84-0.99]). All cases, that developed PE several months later (onset 257.4 ± 15.2 g days), were correctly identified. The model's specificity was 80% [95%CI 65-100] and the overall accuracy was 88% [95%CI 73-95]. Incorporating additionally the placental FLT1 rs4769613 T/C genotype data increased the prediction accuracy to 93.5% [AUC = 0.97 (95%CI 0.89-1.00)]. However, 6PLEX measurements of samples collected at 100-182 g days were insufficiently informative to develop reliable PE prediction models for mid-pregnancy (accuracy <75%). In summary, the developed model opens new horizons for first-trimester PE screening, combining the easily standardizable 6PLEX assay with routinely collected antenatal care data and resulting in high sensitivity and specificity.
Keywords: 6PLEX; PTX3; Preeclampsia; biomarkers; early pregnancy; multiplex immunoassay; risk prediction; statistical modeling.
Copyright © 2022 Ratnik, Rull, Aasmets, Kikas, Hanson, Kisand, Fischer and Laan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

