Population-wide persistent hemostatic changes after vaccination with ChAdOx1-S
- PMID: 35966540
- PMCID: PMC9372359
- DOI: 10.3389/fcvm.2022.966028
Population-wide persistent hemostatic changes after vaccination with ChAdOx1-S
Abstract
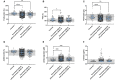
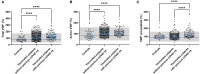
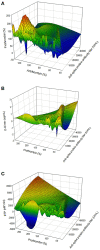
Various vaccines were developed to reduce the spread of the Severe Acute Respiratory Syndrome Cov-2 (SARS-CoV-2) virus. Quickly after the start of vaccination, reports emerged that anti-SARS-CoV-2 vaccines, including ChAdOx1-S, could be associated with an increased risk of thrombosis. We investigated the hemostatic changes after ChAdOx1-S vaccination in 631 health care workers. Blood samples were collected 32 days on average after the second ChAdOx1-S vaccination, to evaluate hemostatic markers such as D-dimer, fibrinogen, α2-macroglobulin, FVIII and thrombin generation. Endothelial function was assessed by measuring Von Willebrand Factor (VWF) and active VWF. IL-6 and IL-10 were measured to study the activation of the immune system. Additionally, SARS-CoV-2 anti-nucleoside and anti-spike protein antibody titers were determined. Prothrombin and fibrinogen levels were significantly reduced after vaccination (-7.5% and -16.9%, p < 0.0001). Significantly more vaccinated subjects were outside the normal range compared to controls for prothrombin (42.1% vs. 26.4%, p = 0.026) and antithrombin (23.9% vs. 3.6%, p = 0.0010). Thrombin generation indicated a more procoagulant profile, characterized by a significantly shortened lag time (-11.3%, p < 0.0001) and time-to-peak (-13.0% and p < 0.0001) and an increased peak height (32.6%, p = 0.0015) in vaccinated subjects compared to unvaccinated controls. Increased VWF (+39.5%, p < 0.0001) and active VWF levels (+24.1 %, p < 0.0001) pointed toward endothelial activation, and IL-10 levels were significantly increased (9.29 pg/mL vs. 2.43 pg/mL, p = 0.032). The persistent increase of IL-10 indicates that the immune system remains active after ChAdOx1-S vaccination. This could trigger a pathophysiological mechanism causing an increased thrombin generation profile and vascular endothelial activation, which could subsequently result in and increased risk of thrombotic events.
Keywords: COVID-19; ChAdOx1-S; hemostasis; thrombin generation; vaccination.
Copyright © 2022 de Laat, Stragier, de Laat-Kremers, Ninivaggi, Mesotten, Thiessen, Van Pelt, Roest, Penders, Vanelderen, Huskens, De Jongh, Laenen, Fivez, ten Cate, Heylen, Heylen and Steensels.
Conflict of interest statement
BL, RdL-K, MR, DH, and MN are employees of Synapse Research Institute, part of Diagnostica Stago. HC received funding for research from Bayer and Pfizer; compensation fees for consultancy and advisory boards from Daaichi, Pfizer, Leo, Bayer, Galapagos, Anthos, Alexion, and Alveron; shareholder from Coagulation profile; all benefits were transferred to the CARIM institute to support investigator-initiated research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
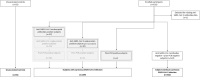


References
-
- J Medicine, JHU . COVID-19 Dashboard by the Center for Systems Science Engineering (CSSE) at Johns Hopkins University (JHU) (2020). Available online at: https://coronavirus.jhu.edu/map.html
LinkOut - more resources
Full Text Sources
Miscellaneous

