Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke
- PMID: 35966580
- PMCID: PMC9373807
- DOI: 10.7150/thno.73931
Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke
Abstract
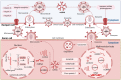
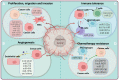
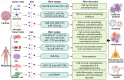
Hypoxia is a central pathophysiological component in cancer, myocardial infarction and ischemic stroke, which represent the most common medical conditions resulting in long-term disability and death. Recent evidence suggests common signaling pathways in these diverse settings mediated by non-coding RNAs (ncRNAs), which are packaged in extracellular vesicles (EVs) protecting ncRNAs from degradation. EVs are a heterogeneous group of lipid bilayer-covered vesicles released from virtually all cells, which have important roles in intercellular communication. Recent studies pointed out that ncRNAs including long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) are selectively sorted into EVs, modulating specific aspects of cancer development, namely cell proliferation, migration, invasion, angiogenesis, immune tolerance or drug resistance, under conditions of hypoxia in recipient cells. In myocardial infarction and stroke, ncRNAs shuttled via EVs have been shown to control tissue survival and remodeling post-hypoxia by regulating cell injury, inflammatory responses, angiogenesis, neurogenesis or neuronal plasticity. This review discusses recent evidence on EV-associated ncRNAs in hypoxic cancer, myocardial infarction and stroke, discussing their cellular origin, biological function and disease significance. The emerging concept of lncRNA-circular RNA/ miRNA/ mRNA networks is outlined, upon which ncRNAs synergistically respond to hypoxia in order to modify disease responses. Particular notion is given to ncRNAs participating in at least two of the three conditions, which revealed a large degree of overlaps across pathophysiological conditions. Possible roles of EV-ncRNAs as therapeutic products or theranostic markers are defined.
© The author(s).
Conflict of interest statement
Competing Interests: D.M.H., B.G. and T.R.D. hold a patent on extracellular vesicles for the treatment of inflammatory conditions (US9877989B2).
Figures

References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP. et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. - PubMed
-
- Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203:1253–63. - PubMed
-
- Fandrey J, Gassmann M. Oxygen sensing and the activation of the hypoxia inducible factor 1 (HIF-1)-invited article. Adv Exp Med Biol. 2009;648:197–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

