Therapeutic aptamer targeting sclerostin loop3 for promoting bone formation without increasing cardiovascular risk in osteogenesis imperfecta mice
- PMID: 35966595
- PMCID: PMC9373813
- DOI: 10.7150/thno.63177
Therapeutic aptamer targeting sclerostin loop3 for promoting bone formation without increasing cardiovascular risk in osteogenesis imperfecta mice
Abstract
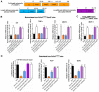
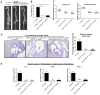
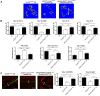
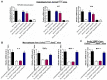
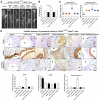
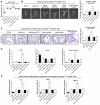
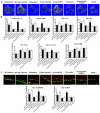
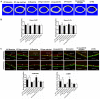
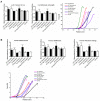
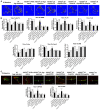
Rationale: Sclerostin inhibition demonstrated bone anabolic potential in osteogenesis imperfecta (OI) mice, whereas humanized therapeutic sclerostin antibody romosozumab for postmenopausal osteoporosis imposed clinically severe cardiac ischemic events. Therefore, it is desirable to develop the next generation sclerostin inhibitors to promote bone formation without increasing cardiovascular risk for OI. Methods and Results: Our data showed that sclerostin suppressed inflammatory responses, prevented aortic aneurysm (AA) and atherosclerosis progression in hSOSTki.Col1a2+/G610C.ApoE-/- mice. Either loop2&3 deficiency or inhibition attenuated sclerostin's suppressive effects on expression of inflammatory cytokines and chemokines in vitro, whilst loop3 deficiency maintained the protective effect of sclerostin on cardiovascular system both in vitro and in vivo. Moreover, loop3 was critical for sclerostin's antagonistic effect on bone formation in Col1a2+/G610C mice. Accordingly, a sclerostin loop3-specific aptamer aptscl56 was identified by our lab. It could recognize both recombinant sclerostin and sclerostin in the serum of OI patients via targeting loop3. PEG40k conjugated aptscl56 (Apc001PE) demonstrated to promote bone formation, increase bone mass and improve bone microarchitecture integrity in Col1a2+/G610C mice via targeting loop3, while did not show influence in inflammatory response, AA and atherosclerosis progression in Col1a2+/G610C.ApoE-/- mice with Angiotensin II infusion. Further, Apc001PE had no influence in the protective effect of sclerostin on cardiovascular system in hSOSTki.Col1a2+/G610C.ApoE-/- mice, while it inhibited the antagonistic effect of sclerostin on bone formation in hSOSTki.Col1a2+/G610C mice via targeting loop3. Apc001PE was non-toxic to healthy rodents, even at ultrahigh dose. Apc001PE for OI was granted orphan drug designation by US-FDA in 2019 (DRU-2019-6966). Conclusion: Sclerostin loop3-specific aptamer Apc001PE promoted bone formation without increasing cardiovascular risk in OI mice.
Keywords: Aptamer; bone formation; no cardiovascular risk; no toxicity; osteogenesis imperfecta; sclerostin loop3.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Role of Sclerostin in Cardiovascular System.Int J Mol Sci. 2025 May 9;26(10):4552. doi: 10.3390/ijms26104552. Int J Mol Sci. 2025. PMID: 40429697 Free PMC article. Review.
-
Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation.Nat Commun. 2022 Jul 22;13(1):4241. doi: 10.1038/s41467-022-31997-8. Nat Commun. 2022. PMID: 35869074 Free PMC article.
-
Sclerostin inhibition in rare bone diseases: Molecular understanding and therapeutic perspectives.J Orthop Translat. 2024 Jun 19;47:39-49. doi: 10.1016/j.jot.2024.05.004. eCollection 2024 Jul. J Orthop Translat. 2024. PMID: 39007037 Free PMC article. Review.
-
Bone mineral properties in growing Col1a2(+/G610C) mice, an animal model of osteogenesis imperfecta.Bone. 2016 Jun;87:120-9. doi: 10.1016/j.bone.2016.04.011. Epub 2016 Apr 13. Bone. 2016. PMID: 27083399 Free PMC article.
-
Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta.J Bone Miner Res. 2014 Oct;29(10):2297-306. doi: 10.1002/jbmr.2198. J Bone Miner Res. 2014. PMID: 24677211 Free PMC article.
Cited by
-
Investigation of the stent induced deformation on hemodynamic of internal carotid aneurysms by computational fluid dynamics.Sci Rep. 2023 May 2;13(1):7155. doi: 10.1038/s41598-023-34383-6. Sci Rep. 2023. PMID: 37130902 Free PMC article.
-
Role of Sclerostin in Cardiovascular System.Int J Mol Sci. 2025 May 9;26(10):4552. doi: 10.3390/ijms26104552. Int J Mol Sci. 2025. PMID: 40429697 Free PMC article. Review.
-
Strategies for developing long-lasting therapeutic nucleic acid aptamer targeting circulating protein: The present and the future.Front Cell Dev Biol. 2022 Nov 1;10:1048148. doi: 10.3389/fcell.2022.1048148. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36393853 Free PMC article. Review.
-
Association of prognostic nutritional index with mortalities in American adult cancer survivors: A cohort study based on NHANES, 1999-2018.Food Sci Nutr. 2023 Nov 30;12(3):1834-1846. doi: 10.1002/fsn3.3877. eCollection 2024 Mar. Food Sci Nutr. 2023. PMID: 38455180 Free PMC article.
-
A Survey of COVID-19 Diagnosis Using Routine Blood Tests with the Aid of Artificial Intelligence Techniques.Diagnostics (Basel). 2023 May 16;13(10):1749. doi: 10.3390/diagnostics13101749. Diagnostics (Basel). 2023. PMID: 37238232 Free PMC article. Review.
References
-
- Marini JC, Forlino A, Bachinger HP, Bishop NJ, Byers PH, Paepe A. et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017;3:17052. - PubMed
-
- Holdsworth G, Roberts SJ, Ke HZ. Novel actions of sclerostin on bone. J Mol Endocrinol. 2019;62:R167–R85. - PubMed
-
- Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S. et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016;375:1532–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

