Asymmetric Synthesis of Nortropanes via Rh-Catalyzed Allylic Arylation
- PMID: 35966601
- PMCID: PMC9361292
- DOI: 10.1021/acscatal.2c02259
Asymmetric Synthesis of Nortropanes via Rh-Catalyzed Allylic Arylation
Abstract
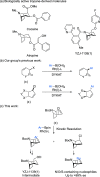
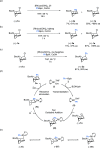
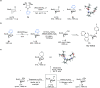
Tropane derivatives are extensively used in medicine, but catalytic asymmetric methods for their synthesis are underexplored. Here, we report Rh-catalyzed asymmetric Suzuki-Miyaura-type cross-coupling reactions between a racemic N-Boc-nortropane-derived allylic chloride and (hetero)aryl boronic esters. The reaction proceeds via an unexpected kinetic resolution, and the resolved enantiopure allyl chloride can undergo highly enantiospecific reactions with N-, O-, and S-containing nucleophiles. The method was applied in a highly stereoselective formal synthesis of YZJ-1139(1), a potential insomnia treatment that recently completed Phase II clinical trials. Our report represents an asymmetric catalytic method for the synthesis of YZJ-1139(1) and related compounds.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Oxford University Innovation has filed a patent application(PCT/GB2016/051612) with S.P.F. named as an inventor. Y.Z., F.W.G. and K.E.C. declare no competing financial interests.
Figures

Similar articles
-
Scale-Up of a Rh-Catalyzed Asymmetric sp3-sp2 Suzuki-Miyaura-Type Reaction.Org Process Res Dev. 2022 Nov 18;26(11):3153-3160. doi: 10.1021/acs.oprd.2c00268. Epub 2022 Nov 2. Org Process Res Dev. 2022. PMID: 36437898 Free PMC article. Review.
-
Highly Enantioselective Hiyama Cross-Coupling via Rh-Catalyzed Allylic Arylation of Racemic Allyl Chlorides.Organometallics. 2019 Oct 28;38(20):3991-3995. doi: 10.1021/acs.organomet.9b00197. Epub 2019 May 10. Organometallics. 2019. PMID: 32055086 Free PMC article.
-
Enantio- and Diastereoselective Suzuki-Miyaura Coupling with Racemic Bicycles.Angew Chem Int Ed Engl. 2019 Aug 26;58(35):12128-12132. doi: 10.1002/anie.201906478. Epub 2019 Jul 25. Angew Chem Int Ed Engl. 2019. PMID: 31246358 Free PMC article.
-
Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids.Nat Chem. 2015 Nov;7(11):935-9. doi: 10.1038/nchem.2360. Epub 2015 Oct 12. Nat Chem. 2015. PMID: 26492015
-
Applications of Transition-Metal-Catalyzed Asymmetric Allylic Substitution in Total Synthesis of Natural Products: An Update.Chem Rec. 2021 Jan;21(1):29-68. doi: 10.1002/tcr.202000086. Epub 2020 Nov 18. Chem Rec. 2021. PMID: 33206466 Review.
Cited by
-
Scale-Up of a Rh-Catalyzed Asymmetric sp3-sp2 Suzuki-Miyaura-Type Reaction.Org Process Res Dev. 2022 Nov 18;26(11):3153-3160. doi: 10.1021/acs.oprd.2c00268. Epub 2022 Nov 2. Org Process Res Dev. 2022. PMID: 36437898 Free PMC article. Review.
-
Rh(I)-Catalyzed Regio- and Enantioselective Ring Opening of Vinyl Cyclopropanes.J Am Chem Soc. 2024 Sep 4;146(35):24708-24715. doi: 10.1021/jacs.4c09490. Epub 2024 Aug 20. J Am Chem Soc. 2024. PMID: 39163089 Free PMC article.
-
Rh(I)-Catalyzed Modular Synthesis of Axially Chiral Alkylidene Azacycloalkanes.ACS Cent Sci. 2025 May 11;11(6):899-909. doi: 10.1021/acscentsci.5c00232. eCollection 2025 Jun 25. ACS Cent Sci. 2025. PMID: 40585798 Free PMC article.
-
Chelation enables selectivity control in enantioconvergent Suzuki-Miyaura cross-couplings on acyclic allylic systems.Nat Chem. 2024 May;16(5):791-799. doi: 10.1038/s41557-023-01430-8. Epub 2024 Feb 8. Nat Chem. 2024. PMID: 38332329 Free PMC article.
References
-
- Afewerki S.; Wang J.-X.; Liao W.-W.; Córdova A.. The Chemical Synthesis and Applications of Tropane Alkaloids. In The Alkaloids: Chemistry and Biology,Knölker H.-J., Ed.; Academic Press, 2019; Vol. 81, pp 151–233. - PubMed
-
- Osbourn A. E.; Lanzotti V.. Plant-Derived Natural Products; Springer, 2009.
LinkOut - more resources
Full Text Sources
