Asymmetric Synthesis of Nortropanes via Rh-Catalyzed Allylic Arylation
- PMID: 35966601
- PMCID: PMC9361292
- DOI: 10.1021/acscatal.2c02259
Asymmetric Synthesis of Nortropanes via Rh-Catalyzed Allylic Arylation
Abstract
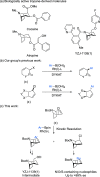
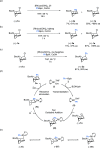
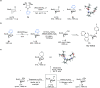
Tropane derivatives are extensively used in medicine, but catalytic asymmetric methods for their synthesis are underexplored. Here, we report Rh-catalyzed asymmetric Suzuki-Miyaura-type cross-coupling reactions between a racemic N-Boc-nortropane-derived allylic chloride and (hetero)aryl boronic esters. The reaction proceeds via an unexpected kinetic resolution, and the resolved enantiopure allyl chloride can undergo highly enantiospecific reactions with N-, O-, and S-containing nucleophiles. The method was applied in a highly stereoselective formal synthesis of YZJ-1139(1), a potential insomnia treatment that recently completed Phase II clinical trials. Our report represents an asymmetric catalytic method for the synthesis of YZJ-1139(1) and related compounds.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Oxford University Innovation has filed a patent application(PCT/GB2016/051612) with S.P.F. named as an inventor. Y.Z., F.W.G. and K.E.C. declare no competing financial interests.
Figures

References
-
- Afewerki S.; Wang J.-X.; Liao W.-W.; Córdova A.. The Chemical Synthesis and Applications of Tropane Alkaloids. In The Alkaloids: Chemistry and Biology,Knölker H.-J., Ed.; Academic Press, 2019; Vol. 81, pp 151–233. - PubMed
-
- Osbourn A. E.; Lanzotti V.. Plant-Derived Natural Products; Springer, 2009.
LinkOut - more resources
Full Text Sources
