Visual Detection of COVID-19 from Materials Aspect
- PMID: 35966612
- PMCID: PMC9358106
- DOI: 10.1007/s42765-022-00179-y
Visual Detection of COVID-19 from Materials Aspect
Abstract
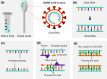
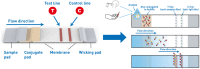
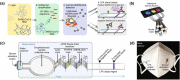
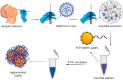
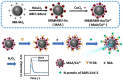
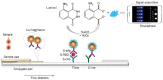
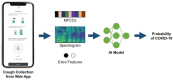
Abstract: In the recent COVID-19 pandemic, World Health Organization emphasized that early detection is an effective strategy to reduce the spread of SARS-CoV-2 viruses. Several diagnostic methods, such as reverse transcription-polymerase chain reaction (RT-PCR) and lateral flow immunoassay (LFIA), have been applied based on the mechanism of specific recognition and binding of the probes to viruses or viral antigens. Although the remarkable progress, these methods still suffer from inadequate cellular materials or errors in the detection and sampling procedure of nasopharyngeal/oropharyngeal swab collection. Therefore, developing accurate, ultrafast, and visualized detection calls for more advanced materials and technology urgently to fight against the epidemic. In this review, we first summarize the current methodologies for SARS-CoV-2 diagnosis. Then, recent representative examples are introduced based on various output signals (e.g., colorimetric, fluorometric, electronic, acoustic). Finally, we discuss the limitations of the methods and provide our perspectives on priorities for future test development.
Keywords: COVID-19; Materials aspects; Rapid and high-throughput detection; Virus diagnosis; Visual detection.
© Donghua University, Shanghai, China 2022.
Conflict of interest statement
Conflict of interestThere are no conflicts of interest.
Figures













Similar articles
-
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications.Microbiol Spectr. 2024 Jun 4;12(6):e0007324. doi: 10.1128/spectrum.00073-24. Epub 2024 Apr 29. Microbiol Spectr. 2024. PMID: 38683014 Free PMC article.
-
A colorimetric lateral flow immunoassay based on oriented antibody immobilization for sensitive detection of SARS-CoV-2.Sens Actuators B Chem. 2023 Mar 15;379:133245. doi: 10.1016/j.snb.2022.133245. Epub 2022 Dec 26. Sens Actuators B Chem. 2023. PMID: 36589904 Free PMC article.
-
Gold Nanoparticle-Based Colorimetric and Fluorescent Dual-Mode Lateral Flow Immunoassay for SARS-CoV-2 Detection.J Funct Biomater. 2024 Feb 27;15(3):58. doi: 10.3390/jfb15030058. J Funct Biomater. 2024. PMID: 38535251 Free PMC article.
-
Laboratory-Based Resources for COVID-19 Diagnostics: Traditional Tools and Novel Technologies. A Perspective of Personalized Medicine.J Pers Med. 2021 Jan 13;11(1):42. doi: 10.3390/jpm11010042. J Pers Med. 2021. PMID: 33451039 Free PMC article. Review.
-
Development and Efficacy of Lateral Flow Point-of-Care Testing Devices for Rapid and Mass COVID-19 Diagnosis by the Detections of SARS-CoV-2 Antigen and Anti-SARS-CoV-2 Antibodies.Diagnostics (Basel). 2021 Sep 24;11(10):1760. doi: 10.3390/diagnostics11101760. Diagnostics (Basel). 2021. PMID: 34679458 Free PMC article. Review.
Cited by
-
Electroactive nanofibrous membrane with temperature monitoring for wound healing.RSC Adv. 2023 May 10;13(21):14224-14235. doi: 10.1039/d3ra01665j. eCollection 2023 May 9. RSC Adv. 2023. PMID: 37179989 Free PMC article.
-
The application of tissue engineering strategies for uterine regeneration.Mater Today Bio. 2025 Feb 18;31:101594. doi: 10.1016/j.mtbio.2025.101594. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40070871 Free PMC article. Review.
-
Elastic Fibers/Fabrics for Wearables and Bioelectronics.Adv Sci (Weinh). 2022 Dec;9(35):e2203808. doi: 10.1002/advs.202203808. Epub 2022 Oct 17. Adv Sci (Weinh). 2022. PMID: 36253094 Free PMC article. Review.
-
Research progress in preparation, properties, and applications of medical protective fiber materials.Appl Mater Today. 2023 Jun;32:101792. doi: 10.1016/j.apmt.2023.101792. Epub 2023 Mar 10. Appl Mater Today. 2023. PMID: 36937335 Free PMC article. Review.
-
Regulating the microenvironment with nanomaterials: Potential strategies to ameliorate COVID-19.Acta Pharm Sin B. 2023 Feb 21;13(9):3638-58. doi: 10.1016/j.apsb.2023.02.010. Online ahead of print. Acta Pharm Sin B. 2023. PMID: 36846153 Free PMC article. Review.
References
-
- Scientists in our country have discovered a new drug for the treatment of the coronavirus and obtained the authorization of the invention patent. Sci Tech Daily. 2022. http://digitalpaper.stdaily.com/http_www.kjrb.com/kjrb/html/2022-05/13/n.... Accessed 20 May 2022.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
