Intensity and longevity of SARS-CoV-2 vaccination response in patients with immune-mediated inflammatory disease: a prospective cohort study
- PMID: 35966645
- PMCID: PMC9363042
- DOI: 10.1016/S2665-9913(22)00191-6
Intensity and longevity of SARS-CoV-2 vaccination response in patients with immune-mediated inflammatory disease: a prospective cohort study
Abstract
Background: Concerns have been raised about the reduced immunogenicity of vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory diseases and the higher risk of breakthrough infections. The objective of our study was to investigate the intensity and longevity of SARS-CoV-2 vaccination responses in patients with immune-mediated inflammatory diseases, and to assess the effects of diagnosis, treatment, and adapted vaccination schedules.
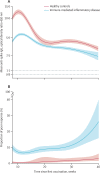
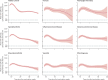
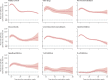
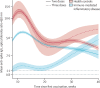
Methods: SARS-CoV-2 IgG antibody response after SARS-CoV-2 vaccination was measured over time in a large prospective cohort of healthy controls and participants with immune-mediated inflammatory diseases (attending or admitted to affiliated centres) between Dec 15, 2020, and Dec 1, 2021. Cohort participants with immune-mediated inflammatory diseases and control participants with no diagnosis of immune-mediated inflammatory diseases, were eligible for this analysis. Demographic data and disease-specific data were collected using a questionnaire. Humoral response was compared across treatment and disease groups, and with respect to the receipt of additional vaccinations. SARS-CoV-2 antibody response was measured by ELISA using optical density ratio units and modelled over time with age and sex adjustment using mixed-effects models. Using these models, marginal mean antibody titres and marginal risks of a poor response (optical density ratio <1·1) were calculated for each week starting from week 8 after the first vaccination to week 40.
Findings: Among 5076 individuals registered, 2535 participants with immune-mediated inflammatory diseases (mean age 55·0 [15·2] years; 1494 [58·9%] women and 1041 [41·1%] men) and 1198 healthy controls (mean age 40·7 [13·5] years; 554 [46·2%] women and 644 [53·8%] men) were included in this analysis. Mean antibody titres were higher in healthy controls compared with people with immune-mediated inflammatory diseases at all timepoints, with a peak antibody response in healthy controls (mean optical density ratio 12·48; 95% CI 11·50-13·53) of more than twice that in participants with immune-mediated inflammatory diseases (5·50; 5·23-5·77; mean difference 6·98; 5·92-8·04). A poor response to vaccination was observed in participants with immune-mediated inflammatory diseases who were taking B-cell inhibitors (peak mean difference from healthy controls 11·68; 10·07-13·29) and T-cell inhibitors (peakmean difference from healthy controls 10·43; 8·33-12·53). Mean differences in antibody responses between different immune-mediated inflammatory diseases were small. Participants with immune-mediated inflammatory diseases who were given a third vaccine dose had higher mean antibody titres than did healthy controls vaccinated with two vaccine doses at 40 weeks after the initial vaccination (mean difference 1·34; 0·01-2·69).
Interpretation: People with immune-mediated inflammatory diseases show a lower and less durable SARS-CoV-2 vaccination response and are at risk of losing humoral immune protection. Adjusted vaccination schedules with earlier booster doses or more frequent re-doses, or both, could better protect people with immune-mediated inflammatory diseases.
Funding: Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, European Research Council, Innovative Medicine Initiative, Friedrich-Alexander-Universität Erlangen-Nürnberg, Else Kröner-Memorial Foundation.
© 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CB reports honorarium for lectures from Almirall Hermal, LEO Pharma, and Novartis Pharma; and participation on a data safety monitoring board or advisory board for Almirall Hermal and Novartis Pharma. MS reports royalties from Becton Dickinson and BioRad; honorarium for lectures from Abbvie, Amgen, Boehringer Ingelheim, Celgene, Janssen-Cilag, Leo, Pfizer, Merck Sharpe & Dohme, and Novartis; and participation on a data safety monitoring board or advisory board for Abbvie, Amgen, Celgene, Janssen-Cilag, Lilly, Pfizer, Merck Sharpe & Dohme, Novartis, Leo, Sanofi, and Union Chimique Belge. MFN reports consultancy fees from the British Medical Association house, Janssen-Cilag, Pentax, and S Karger; and honorarium for lectures from Asian Organisation for Crohn's and Colitis, Falk foundation, Janssen-Cilag, Lilly Deutschland, Medi K, Northwell Foundation, Scherl-Roberts, Skaggs School of Pharmacy and Pharmaceutical sciences, and Takeda Pharmaceuticals International. All other authors declare no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

