Distribution and inter-regional relationship of amyloid-beta plaque deposition in a 5xFAD mouse model of Alzheimer's disease
- PMID: 35966777
- PMCID: PMC9371463
- DOI: 10.3389/fnagi.2022.964336
Distribution and inter-regional relationship of amyloid-beta plaque deposition in a 5xFAD mouse model of Alzheimer's disease
Abstract
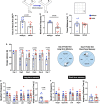
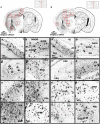
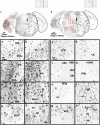
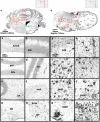
Alzheimer's disease (AD) is the most common form of dementia. Although previous studies have selectively investigated the localization of amyloid-beta (Aβ) deposition in certain brain regions, a comprehensive characterization of the rostro-caudal distribution of Aβ plaques in the brain and their inter-regional correlation remain unexplored. Our results demonstrated remarkable working and spatial memory deficits in 9-month-old 5xFAD mice compared to wildtype mice. High Aβ plaque load was detected in the somatosensory cortex, piriform cortex, thalamus, and dorsal/ventral hippocampus; moderate levels of Aβ plaques were observed in the motor cortex, orbital cortex, visual cortex, and retrosplenial dysgranular cortex; and low levels of Aβ plaques were located in the amygdala, and the cerebellum; but no Aβ plaques were found in the hypothalamus, raphe nuclei, vestibular nucleus, and cuneate nucleus. Interestingly, the deposition of Aβ plaques was positively associated with brain inter-regions including the prefrontal cortex, somatosensory cortex, medial amygdala, thalamus, and the hippocampus. In conclusion, this study provides a comprehensive morphological profile of Aβ deposition in the brain and its inter-regional correlation. This suggests an association between Aβ plaque deposition and specific brain regions in AD pathogenesis.
Keywords: 5xFAD; Alzheimer’s disease; amyloid-beta (AB); dementia; morphology; neuroanatomy.
Copyright © 2022 Tsui, Roy, Chau, Wong, Shi, Poon, Wang, Strekalova, Aquili, Chang, Fung, Song and Lim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Butyrylcholinesterase-knockout reduces fibrillar β-amyloid and conserves 18FDG retention in 5XFAD mouse model of Alzheimer's disease.Brain Res. 2017 Sep 15;1671:102-110. doi: 10.1016/j.brainres.2017.07.009. Epub 2017 Jul 17. Brain Res. 2017. PMID: 28729192
-
Early Detection of Aβ Deposition in the 5xFAD Mouse by Amyloid PET.Contrast Media Mol Imaging. 2018 Feb 28;2018:5272014. doi: 10.1155/2018/5272014. eCollection 2018. Contrast Media Mol Imaging. 2018. PMID: 29681782 Free PMC article.
-
β-Amyloid, cholinergic transmission, and cerebrovascular system -- a developmental study in a mouse model of Alzheimer's disease.Curr Pharm Des. 2013;19(38):6749-65. doi: 10.2174/13816128113199990711. Curr Pharm Des. 2013. PMID: 23530514
-
Amyloid Deposition and Dendritic Complexity of Corticocortical Projection Cells in Five Familial Alzheimer's Disease Mouse.Neuroscience. 2023 Feb 21;512:85-98. doi: 10.1016/j.neuroscience.2022.12.013. Epub 2022 Dec 19. Neuroscience. 2023. PMID: 36549605 Free PMC article.
-
Commonalities between Copper Neurotoxicity and Alzheimer's Disease.Toxics. 2021 Jan 7;9(1):4. doi: 10.3390/toxics9010004. Toxics. 2021. PMID: 33430181 Free PMC article. Review.
Cited by
-
Preserved blood-brain barrier and neurovascular coupling in female 5xFAD model of Alzheimer's disease.Front Aging Neurosci. 2023 May 5;15:1089005. doi: 10.3389/fnagi.2023.1089005. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37261266 Free PMC article.
-
Intermittent theta burst stimulation attenuates oxidative stress and reactive astrogliosis in the streptozotocin-induced model of Alzheimer's disease-like pathology.Front Aging Neurosci. 2023 May 18;15:1161678. doi: 10.3389/fnagi.2023.1161678. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37273654 Free PMC article.
-
KChIP3 fosters neuroinflammation and synaptic dysfunction in the 5XFAD mouse model of Alzheimer's disease.J Neuroinflammation. 2025 Jun 19;22(1):160. doi: 10.1186/s12974-025-03426-2. J Neuroinflammation. 2025. PMID: 40537787 Free PMC article.
-
Detecting the effect of genetic diversity on brain composition in an Alzheimer's disease mouse model.Commun Biol. 2024 May 20;7(1):605. doi: 10.1038/s42003-024-06242-1. Commun Biol. 2024. PMID: 38769398 Free PMC article.
-
Olfactory deficits in aging and Alzheimer's-spotlight on inhibitory interneurons.Front Neurosci. 2024 Dec 16;18:1503069. doi: 10.3389/fnins.2024.1503069. eCollection 2024. Front Neurosci. 2024. PMID: 39737436 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

