Linderalactone Suppresses Pancreatic Cancer Development In Vitro and In Vivo via Negatively Regulating PI3K/AKT Signaling Pathway
- PMID: 35966890
- PMCID: PMC9371883
- DOI: 10.1155/2022/8675096
Linderalactone Suppresses Pancreatic Cancer Development In Vitro and In Vivo via Negatively Regulating PI3K/AKT Signaling Pathway
Abstract
Linderalactone is one of the main extracts of Linderae Radix, which is widely used in traditional Chinese medicine. There have been few studies on the antitumor effect of linderalactone in the past. In this study, we explored the anti-pancreatic cancer activity of linderalactone in vitro and in vivo. The results showed that linderalactone inhibited the proliferation of pancreatic cancer cells in a time- and dose-dependent manner. Cell migration and invasion were significantly inhibited by linderalactone. The cell cycle was arrested in the G2/M phase, and the expression levels of cell cycle-associated proteins changed significantly with linderalactone treatment. In addition, linderalactone induced cell apoptosis and altered the expression of apoptotic markers, such as caspase 3 and PARP1. Mechanistically, linderalactone suppressed the PI3K/AKT signaling pathway by downregulating the phosphorylation of PI3K and AKT. The xenograft study results were consistent with the in vitro results, and there was no obvious chemical toxicity. Thus, our research demonstrated that linderalactone exhibits antitumor activity against pancreatic cancer and may be developed as a potential anti-pancreatic cancer agent in the future.
Copyright © 2022 Dongchao Xu et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
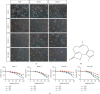
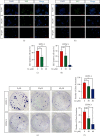



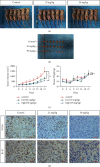
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

