Effectiveness of treatment for 31 patients with seropositive autoimmune autonomic ganglionopathy in Japan
- PMID: 35966941
- PMCID: PMC9364197
- DOI: 10.1177/17562864221110048
Effectiveness of treatment for 31 patients with seropositive autoimmune autonomic ganglionopathy in Japan
Abstract
Background: Autoimmune autonomic ganglionopathy (AAG) is characterized by serum autoantibodies against the ganglionic acetylcholine receptor (gAChR). Immunomodulatory treatments may alleviate AAG symptoms, but the most appropriate treatment strategy is unclear.
Objective: This study aimed to confirm the effectiveness of treatments, particularly immunotherapy, in patients with seropositive AAG in Japan, as well as to determine the most effective treatment and the best assessment method for clinical response to treatment.
Methods: We collected data from a previous cohort study of patients with seropositive AAG. The clinical autonomic and extra-autonomic symptoms were objectively counted and subjectively assessed using the modified Composite Autonomic Symptom Score. Post-treatment changes in the gAChR antibody level were evaluated.
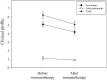
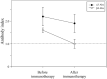
Results: Thirty-one patients received immunotherapy. Among them, 19 patients received intravenous methylprednisolone; 27, intravenous immunoglobulin; 3, plasma exchange; 18, oral steroids; 2, tacrolimus; 1, cyclosporine; and 1, mycophenolate mofetil. Patients who received immunotherapy showed improvements in the total number of symptoms (from 6.2 ± 2.0 to 5.1 ± 2.0) and modified Composite Autonomic Symptom Score (from 37.4 ± 15.3 to 26.6 ± 12.8). Orthostatic intolerance, sicca, and gastrointestinal symptoms were ameliorated by immunotherapy. Immunotherapy decreased the antibody levels (gAChRα3 antibodies, from 2.2 ± 0.4 to 1.9 ± 0.4, p = 0.08; gAChRβ4 antibodies, from 1.6 ± 0.1 to 1.0 ± 0.2, p = 0.002), but antibody levels increased in 10 patients despite immunotherapy. The rate of improvement in the total number of symptoms was higher in patients with combined therapy than in patients with non-combined therapy (70.7% vs 28.6%).
Conclusions: The scores in many items on the rating scale decreased after immunotherapy in patients with seropositive AAG, particularly in the combined immunotherapy group. However, more accurate assessment scales for clinical symptoms and multicenter randomized, placebo-controlled prospective studies are warranted to establish future treatment strategies.
Keywords: autoantibody; autoimmune autonomic ganglionopathy; ganglionic acetylcholine receptor; immunotherapy; treatment.
© The Author(s), 2022.
Conflict of interest statement
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Vernino S, Low PA, Fealey RD, et al. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343: 847–855. - PubMed
-
- Koike H, Watanabe H, Sobue G. The spectrum of immune-mediated autonomic neuropathies: insights from the clinicopathological features. J Neurol Neurosurg Psychiatry 2013; 84: 98–106. - PubMed
LinkOut - more resources
Full Text Sources

