Synthesis, Catalytic GPx-like Activity, and SET Reactions of Conformationally Constrained 2,7-Dialkoxy-Substituted Naphthalene-1,8- peri- diselenides
- PMID: 35967016
- PMCID: PMC9366784
- DOI: 10.1021/acsomega.2c02286
Synthesis, Catalytic GPx-like Activity, and SET Reactions of Conformationally Constrained 2,7-Dialkoxy-Substituted Naphthalene-1,8- peri- diselenides
Abstract
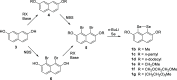
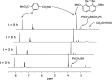
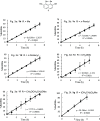
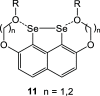
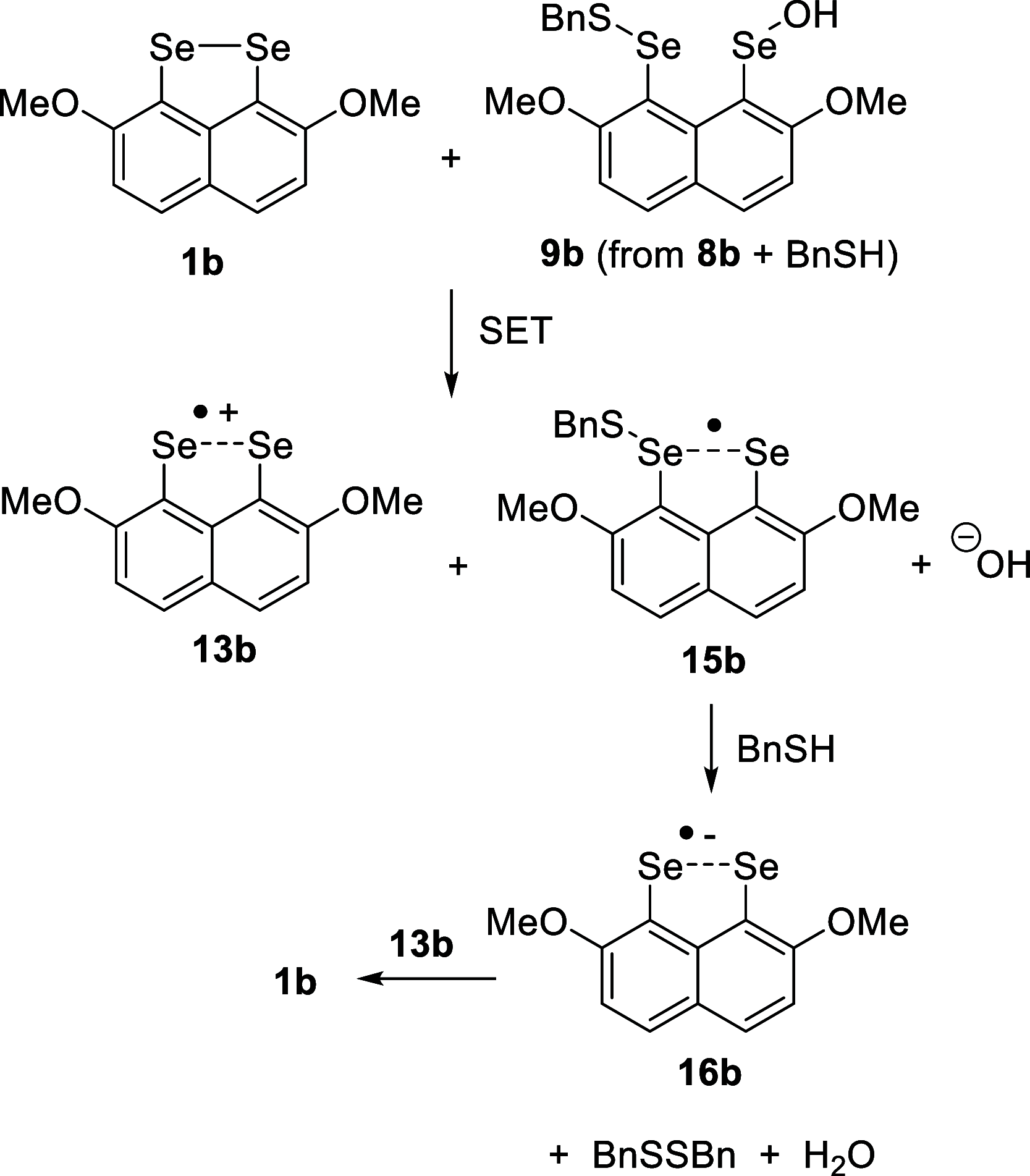
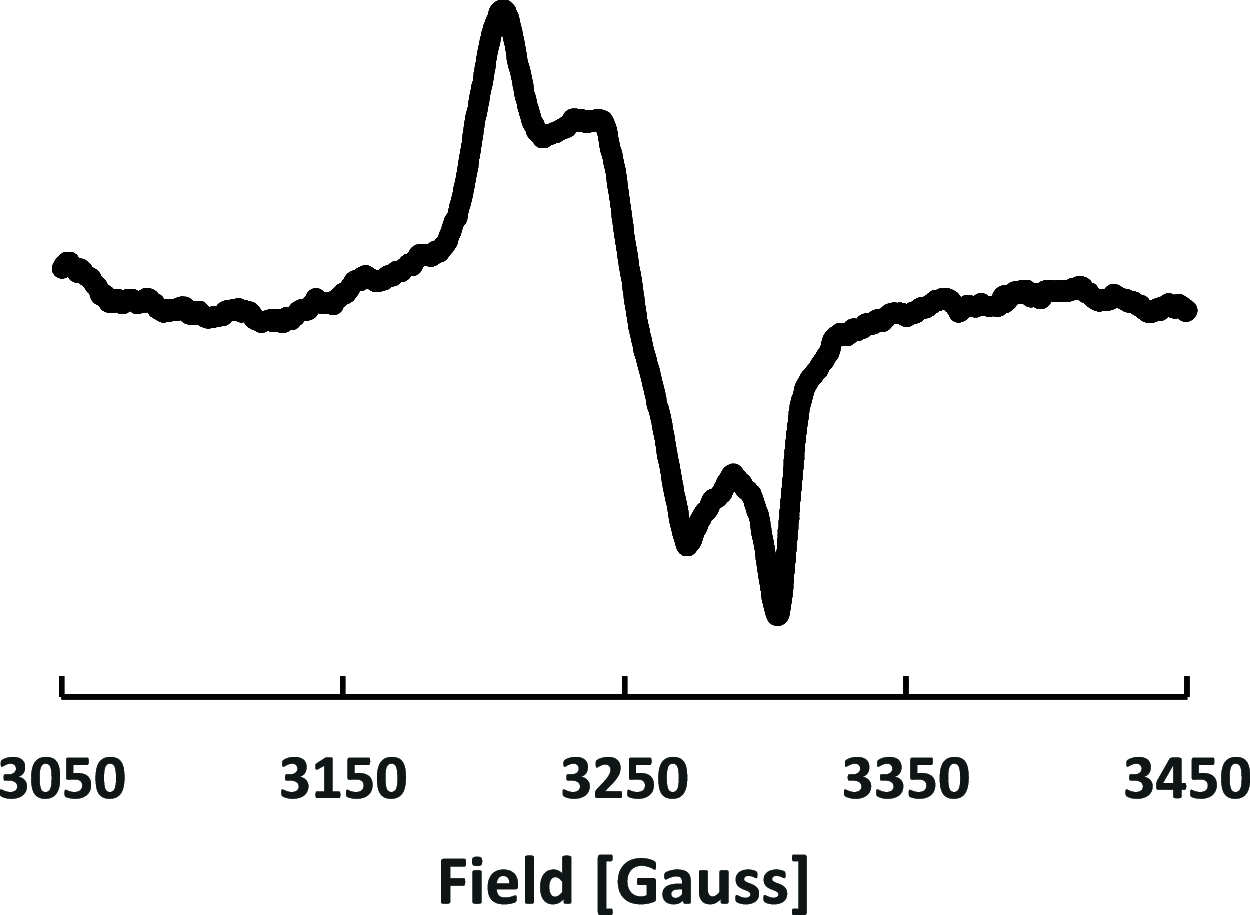
Several 2,7-dialkoxy-substituted naphthalene-1,8-peri-diselenides were prepared and tested for catalytic antioxidant activity in an NMR-based assay employing the reduction of hydrogen peroxide with stoichiometric amounts of benzyl thiol. Acidic conditions enhanced their catalytic activity, whereas basic conditions suppressed it. The highest activity was observed with a 2,7-bis(triethyleneglycol) derivative. These compounds serve as mimetics of the antioxidant selenoenzyme glutathione peroxidase. Studies based on NMR peak-broadening effects and EPR spectroscopy indicated that a thiol-dependent SET reaction occurs under the conditions of the assay, which can be reversed by the addition of triethylamine. In contrast, peak broadening induced by proton-catalyzed electron transfer during the treatment of naphthalene-1,8-peri-diselenides with trifluoroacetic acid can be suppressed by the addition of excess thiol. These observations provide new insights into the redox mechanisms of these processes.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
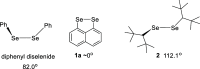

References
-
- Meinwald J.; Dauplaise D.; Wudl F.; Hauser J. J. Peri-bridged Naphthalenes from 1,8-Dilithionaphthalene. J. Am. Chem. Soc. 1977, 99, 255–257. 10.1021/ja00443a051. - DOI
-
- Wudl F.Organoselenium Chemistry; Liotta D., Ed.; John Wiley & Sons: New York, 1987; Chapter 9.
- Ogura F.; Takimiya K.. Organoselenium Chemistry—A Practical Approach; Back T. G., Ed.; Oxford University Press: Oxford, U.K., 1999; Chapter 14.
- Dauplaise D.; Meinwald J.; Scott J. C.; Temkin H.; Clardy J. Synthesis and Properties of Chalcogen-Bridged Naphthalenes: A New Series of Electron Donors. Ann. N.Y. Acad. Sci. 1978, 313, 382–394. 10.1111/j.1749-6632.1978.tb39433.x. - DOI
- Press D. J.; Back T. G.; Sutherland T. C. Charge Transfer Complexes of Electron-Rich Naphthalene Peri-Dichalcogenides with TCNQ. Tetrahedron Lett. 2012, 53, 1603–1605. 10.1016/j.tetlet.2012.01.068. - DOI
-
- Griffin J. M.; Knight F. R.; Hua G.; Ferrara J. S.; Hogan S. W. L.; Woollins J. D.; Ashbrook S. E. 77Se Solid-State NMR of Inorganic and Organoselenium Systems: A Combined Experimental and Computational Study. J. Phys. Chem. C 2011, 115, 10859–10872. 10.1021/jp202550f. - DOI
-
- Aucott S. M.; Milton H. L.; Robertson S. D.; Slawin A. M. Z.; Woollins J. D. Crystal Structures and Molecular Modeling of 1,8-Chalcogenide-Substituted Naphthalenes. Heteroat. Chem. 2004, 15, 530–542. 10.1002/hc.20055. - DOI
- Aucott S. M.; Milton H. L.; Slawin A. M. Z.; Woollins J. D. Crystallographic Studies of Chalcogen Bridged Naphthalenes. Phosphorus, Sulfur Silicon Relat. Elem. 2004, 179, 985.10.1080/10426500490429815. - DOI
-
-
For examples, see:
- Meigh C. B. E.; Nejman P. S.; Slawin A. M. Z.; Derek Woollins J. Complexation of Aromatic Dichalcogen Ligands to Germanium. Inorg. Chim. Acta 2017, 456, 120–127. 10.1016/j.ica.2016.10.038. - DOI
- Aucott S. M.; Milton H. L.; Robertson S. D.; Slawin A. M. Z.; Walker G. D.; Woollins J. D. Platinum Complexes of Naphthalene-1,8-dichalcogen and Related Polyaromatic Hydrocarbon Ligands. Chem.—Eur. J. 2004, 10, 1666–1676. 10.1002/chem.200305352. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Miscellaneous

