Exploring the Cobalt-Histidine Complex for Wide-Ranging Colorimetric O2 Detection
- PMID: 35967046
- PMCID: PMC9366964
- DOI: 10.1021/acsomega.2c03904
Exploring the Cobalt-Histidine Complex for Wide-Ranging Colorimetric O2 Detection
Abstract
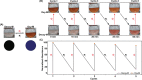
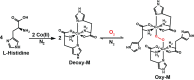
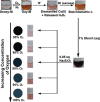
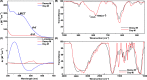
Developing a robust, cost-effective, and user-friendly sensor for monitoring molecular oxygen (O2) ranging from a minute to a medically relevant level (85-100%) in a stream of flowing breathable gas is vital in various industrial domains. Here, we report an innovative application of the cobalt(l-histidine)2 complex, a bioinspired model of O2-carrying metalloproteins, for rapid and reliable sensing of O2 from 0 to 100% saturation levels under realistic conditions. We have established two distinct colorimetric O2 detection techniques, which can be executed with the use of a common smartphone camera and readily available color-detecting software. A series of spectroscopic experiments were performed to demonstrate the molecular-level alteration in cobalt(l-histidine)2 following its exposure to oxygen, leading to an exclusive pink-to-brown color change. Therefore, this study establishes a template for designing bioinspired molecular complexes for O2 sensing, leading to practical and straightforward solutions. This metal-amino acid complex's broad-spectrum sensing of O2 has widened the scope of bioinspired model complexes for divergent applications in industrial sectors.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Self-Sensitized and Reversible O2 Reactivity with Bisphenalenyls for Simple, Tunable, and Multicycle Colorimetric Oxygen-Sensing Films.ACS Appl Mater Interfaces. 2022 Jan 12;14(1):1817-1825. doi: 10.1021/acsami.1c16033. Epub 2021 Dec 27. ACS Appl Mater Interfaces. 2022. PMID: 34958545
-
High-Performance Colorimetric Detection of Thiosulfate by Using Silver Nanoparticles for Smartphone-Based Analysis.ACS Sens. 2017 Aug 25;2(8):1152-1159. doi: 10.1021/acssensors.7b00257. Epub 2017 Aug 2. ACS Sens. 2017. PMID: 28722404
-
Spectroscopic elucidation of the inhibitory mechanism of Cys2His2 zinc finger transcription factors by cobalt(III) Schiff base complexes.Chemistry. 2013 Dec 9;19(50):17043-53. doi: 10.1002/chem.201301659. Epub 2013 Nov 6. Chemistry. 2013. PMID: 24203451 Free PMC article.
-
Recent advances in the fluorimetric and colorimetric detection of cobalt ions.RSC Adv. 2024 Mar 25;14(14):9819-9847. doi: 10.1039/d4ra00445k. eCollection 2024 Mar 20. RSC Adv. 2024. PMID: 38528922 Free PMC article. Review.
-
Inhibition and oxygen activation in copper amine oxidases.Acc Chem Res. 2015 May 19;48(5):1218-26. doi: 10.1021/ar500460z. Epub 2015 Apr 21. Acc Chem Res. 2015. PMID: 25897668 Review.
Cited by
-
A homogeneous cobalt complex mediated electro and photocatalytic O2/H2O interconversion in neutral water.iScience. 2023 Oct 12;26(11):108189. doi: 10.1016/j.isci.2023.108189. eCollection 2023 Nov 17. iScience. 2023. PMID: 37920669 Free PMC article.
-
Advancements in the research of finger-actuated POCT chips.Mikrochim Acta. 2023 Dec 29;191(1):65. doi: 10.1007/s00604-023-06140-z. Mikrochim Acta. 2023. PMID: 38158397 Review.
References
-
- Roberts L.; Lines R.; Reddy S.; Hay J. Investigation of Polyviologens as Oxygen Indicators in Food Packaging. Sens. Actuators, B 2011, 152, 63–67. 10.1016/j.snb.2010.09.047. - DOI
-
- Smolander M.; Hurme E.; Ahvenainen R. Leak Indicators for Modified-Atmosphere Packages. Trends Food Sci. Technol. 1997, 8, 101–106. 10.1016/S0924-2244(97)01017-0. - DOI
LinkOut - more resources
Full Text Sources

