Oxidative Stress: Glutathione and Its Potential to Protect Methionine-35 of Aβ Peptide from Oxidation
- PMID: 35967059
- PMCID: PMC9366984
- DOI: 10.1021/acsomega.2c02760
Oxidative Stress: Glutathione and Its Potential to Protect Methionine-35 of Aβ Peptide from Oxidation
Abstract
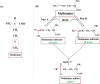
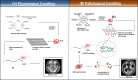
Alzheimer's disease (AD) is the most common neurodegenerative disorder with heterogeneous etiology. Intracellular neurofibrillary tangles caused by tau (τ) protein phosphorylation and extracellular senile plaques caused by aggregation of amyloid-beta (Aβ) peptide are characteristic histopathological hallmarks of AD. Oxidative stress (OS) is also suggested to play a role in the pathophysiology of AD. The antioxidant glutathione (GSH) is able to mitigate OS through the detoxification of free radicals. The clearance of these free radicals is reported to be affected when there is a decline in GSH levels in AD. These radicals further react with the methionine-35 (M-35) residue of Aβ and facilitate its subsequent oligomerization. This review presents a plausible model indicating the role of master antioxidant GSH to protect M35 of Aβ1-40/Aβ1-42 from oxidation in pathological conditions as compared to healthy controls.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

