Radiosensitivity in patients affected by ARPC1B deficiency: a new disease trait?
- PMID: 35967303
- PMCID: PMC9372879
- DOI: 10.3389/fimmu.2022.919237
Radiosensitivity in patients affected by ARPC1B deficiency: a new disease trait?
Abstract
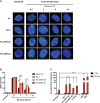
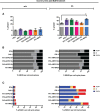
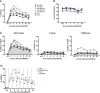
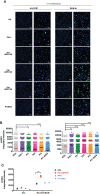
Actin-related protein 2/3 complex subunit 1B (ARPC1B) deficiency is a recently described inborn error of immunity (IEI) presenting with combined immunodeficiency and characterized by recurrent infections and thrombocytopenia. Manifestations of immune dysregulation, including colitis, vasculitis, and severe dermatitis, associated with eosinophilia, hyper-IgA, and hyper-IgE are also described in ARPC1B-deficient patients. To date, hematopoietic stem cell transplantation seems to be the only curative option for patients. ARPC1B is part of the actin-related protein 2/3 complex (Arp2/3) and cooperates with the Wiskott-Aldrich syndrome protein (WASp) in the regulation of the actin cytoskeleton remodeling and in driving double-strand break clustering for homology-directed repair. In this study, we aimed to investigate radiosensitivity (RS) in ARPC1B-deficient patients to assess whether it can be considered an additional disease trait. First, we performed trio-based next-generation-sequencing studies to obtain the ARPC1B molecular diagnosis in our index case characterized by increased RS, and then we confirmed, using three different methods, an increment of radiosensitivity in all enrolled ARPC1B-deficient patients. In particular, higher levels of chromatid-type aberrations and γH2AX foci, with an increased number of cells arrested in the G2/M-phase of the cell cycle, were found in patients' cells after ionizing radiation exposition and radiomimetic bleomycin treatment. Overall, our data suggest increased radiosensitivity as an additional trait in ARPC1B deficiency and support the necessity to investigate this feature in ARPC1B patients as well as in other IEI with cytoskeleton defects to address specific clinical follow-up and optimize therapeutic interventions.
Keywords: ARPC1B; DNA damage response (DDR); combined immunodeficiency; immune dysregulation; radiosensitivity.
Copyright © 2022 Chiriaco, Ursu, Amodio, Cotugno, Volpi, Berardinelli, Pizzi, Cifaldi, Zoccolillo, Prigione, Di Cesare, Giancotta, Anastasio, Rivalta, Pacillo, Zangari, Fiocchi, Diociaiuti, Bruselles, Pantaleoni, Ciolfi, D’Oria, Palumbo, Gattorno, El Hachem, de Villartay, Finocchi, Palma, Rossi, Tartaglia, Aiuti, Antoccia, Di Matteo and Cancrini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer VB declared a shared affiliation with the author J-PV to the handling editor at the time of review.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

