Cystic fibrosis transmembrane regulator correction attenuates heart failure-induced lung inflammation
- PMID: 35967318
- PMCID: PMC9365932
- DOI: 10.3389/fimmu.2022.928300
Cystic fibrosis transmembrane regulator correction attenuates heart failure-induced lung inflammation
Abstract
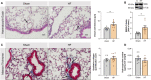
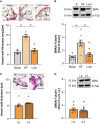
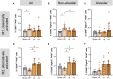
Heart failure (HF) affects 64 million people worldwide. Despite advancements in prevention and therapy, quality of life remains poor for many HF patients due to associated target organ damage. Pulmonary manifestations of HF are well-established. However, difficulties in the treatment of HF patients with chronic lung phenotypes remain as the underlying patho-mechanistic links are still incompletely understood. Here, we aim to investigate the cystic fibrosis transmembrane regulator (CFTR) involvement in lung inflammation during HF, a concept that may provide new mechanism-based therapies for HF patients with pulmonary complications. In a mouse model of HF, pharmacological CFTR corrector therapy (Lumacaftor (Lum)) was applied systemically or lung-specifically for 2 weeks, and the lungs were analyzed using histology, flow cytometry, western blotting, and qPCR. Experimental HF associated with an apparent lung phenotype characterized by vascular inflammation and remodeling, pronounced tissue inflammation as evidenced by infiltration of pro-inflammatory monocytes, and a reduction of pulmonary CFTR+ cells. Moreover, the elevation of a classically-activated phenotype of non-alveolar macrophages coincided with a cell-specific reduction of CFTR expression. Pharmacological correction of CFTR with Lum mitigated the HF-induced downregulation of pulmonary CFTR expression and increased the proportion of CFTR+ cells in the lung. Lum treatment diminished the HF-associated elevation of classically-activated non-alveolar macrophages, while promoting an alternatively-activated macrophage phenotype within the lungs. Collectively, our data suggest that downregulation of CFTR in the HF lung extends to non-alveolar macrophages with consequences for tissue inflammation and vascular structure. Pharmacological CFTR correction possesses the capacity to alleviate HF-associated lung inflammation.
Keywords: cystic fibrosis transmembrane regulator; heart failure; inflammation; lung; macrophages.
Copyright © 2022 Uhl, Vanherle and Meissner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Therapeutic CFTR Correction Normalizes Systemic and Lung-Specific S1P Level Alterations Associated with Heart Failure.Int J Mol Sci. 2022 Jan 14;23(2):866. doi: 10.3390/ijms23020866. Int J Mol Sci. 2022. PMID: 35055052 Free PMC article.
-
Ivacaftor therapy post myocardial infarction augments systemic inflammation and evokes contrasting effects with respect to tissue inflammation in brain and lung.Biomed Pharmacother. 2023 Jun;162:114628. doi: 10.1016/j.biopha.2023.114628. Epub 2023 Apr 3. Biomed Pharmacother. 2023. PMID: 37018991
-
CFTR-dependent defect in alternatively-activated macrophages in cystic fibrosis.J Cyst Fibros. 2017 Jul;16(4):475-482. doi: 10.1016/j.jcf.2017.03.011. Epub 2017 Apr 17. J Cyst Fibros. 2017. PMID: 28428011
-
Lumacaftor/ivacaftor, a novel agent for the treatment of cystic fibrosis patients who are homozygous for the F580del CFTR mutation.Expert Rev Clin Pharmacol. 2017 Oct;10(10):1055-1072. doi: 10.1080/17512433.2017.1378094. Epub 2017 Sep 22. Expert Rev Clin Pharmacol. 2017. PMID: 28891346 Review.
-
Lumacaftor and ivacaftor in the management of patients with cystic fibrosis: current evidence and future prospects.Ther Adv Respir Dis. 2015 Dec;9(6):313-26. doi: 10.1177/1753465815601934. Epub 2015 Sep 28. Ther Adv Respir Dis. 2015. PMID: 26416827 Review.
Cited by
-
Can Bioactive Food Substances Contribute to Cystic Fibrosis-Related Cardiovascular Disease Prevention?Nutrients. 2023 Jan 8;15(2):314. doi: 10.3390/nu15020314. Nutrients. 2023. PMID: 36678185 Free PMC article. Review.
-
Therapeutic CFTR Correction Normalizes Systemic and Lung-Specific S1P Level Alterations Associated with Heart Failure.Int J Mol Sci. 2022 Jan 14;23(2):866. doi: 10.3390/ijms23020866. Int J Mol Sci. 2022. PMID: 35055052 Free PMC article.
References
-
- Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. . Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. a review on behalf of the acute heart failure committee of the heart failure association (Hfa) of the European society of cardiology (Esc). Eur J Heart Fail (2017) 19(7):821–36. doi: 10.1002/ejhf.872 - DOI - PMC - PubMed
-
- Meissner A, Yang J, Kroetsch JT, Sauve M, Dax H, Momen A, et al. . Tumor necrosis factor-Alpha-Mediated downregulation of the cystic fibrosis transmembrane conductance regulator drives pathological sphingosine-1-Phosphate signaling in a mouse model of heart failure. Circulation (2012) 125(22):2739–50. doi: 10.1161/CIRCULATIONAHA.111.047316 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

