Extracellular vesicles from A23187-treated neutrophils cause cGAS-STING-dependent IL-6 production by macrophages
- PMID: 35967325
- PMCID: PMC9374307
- DOI: 10.3389/fimmu.2022.949451
Extracellular vesicles from A23187-treated neutrophils cause cGAS-STING-dependent IL-6 production by macrophages
Abstract
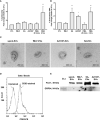
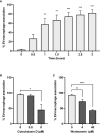
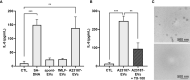
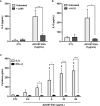
In response to several types of bacteria, as well as pharmacological agents, neutrophils produce extracellular vesicles (EVs) and release DNA in the form of neutrophil extracellular traps (NETs). However, it is unknown whether these two neutrophil products cooperate to modulate inflammation. Consistent with vital NETosis, neutrophils challenged with S. aureus, as well as those treated with A23187, released significantly more DNA relative to untreated or fMLF-treated neutrophils, with no lysis occurring for any condition. To test the hypothesis that EVs generated during NETosis caused macrophage inflammation, we isolated and characterized EVs from A23187-treated neutrophils (A23187-EVs). A23187-EVs associated with neutrophil granule proteins, histone H3, transcription factor A, mitochondrial (TFAM), and nuclear and mitochondrial DNA (mtDNA). We showed that DNA from A23187-EVs, when transfected into macrophages, led to production of IL-6 and IFN-α2, and this response was blunted by pre-treatment with the STING inhibitor H151. Next, we confirmed that A23187-EVs were engulfed by macrophages, and showed that they induced cGAS-STING-dependent IL-6 production. In contrast, neither EVs from untreated or fMLF-treated cells exhibited pro-inflammatory activity. Although detergent-mediated lysis of A23187-EVs diminished IL-6 production, removal of surface-associated DNA with DNase I treatment had no effect, and A23187-EVs did not induce IFN-α2 production. Given these unexpected results, we investigated whether macrophage mtDNA activated the cGAS-STING signaling axis. Consistent with mitochondrial outer membrane permeabilization (MOMP), a defined mechanism of mtDNA release, we observed macrophage mitochondrial membrane depolarization, a decrease in cytosolic Bax, and a decrease in mitochondrial cytochrome c, suggesting that macrophage mtDNA may initiate this EV-dependent signaling cascade. All together, these data demonstrate that A23187-EVs behave differently than transfected NET- or EV-DNA, and that neutrophil-derived EVs could be used as a model to study NF-κB-dependent STING activation.
Keywords: EVs; STING; cGAS; ectosomes; exosomes; inflammation; microvesicles.
Copyright © 2022 Allen, Whitefoot-Keliin, Palmatier, Mahon and Greenlee-Wacker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

