MDA5 signaling induces type 1 IFN- and IL-1-dependent lung vascular permeability which protects mice from opportunistic fungal infection
- PMID: 35967332
- PMCID: PMC9368195
- DOI: 10.3389/fimmu.2022.931194
MDA5 signaling induces type 1 IFN- and IL-1-dependent lung vascular permeability which protects mice from opportunistic fungal infection
Abstract
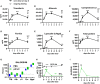
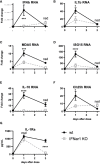
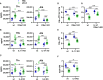
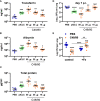
Lungs balance threat from primary viral infection, secondary infection, and inflammatory damage. Severe pulmonary inflammation induces vascular permeability, edema, and organ dysfunction. We previously demonstrated that poly(I:C) (pICLC) induced type 1 interferon (t1IFN) protected mice from Cryptococcus gattii (Cg) via local iron restriction. Here we show pICLC increased serum protein and intravenously injected FITC-dextran in the lung airspace suggesting pICLC induces vascular permeability. Interestingly, pICLC induced a pro-inflammatory signature with significant expression of IL-1 and IL-6 which depended on MDA5 and t1IFN. Vascular permeability depended on MDA5, t1IFN, IL-1, and IL-6. T1IFN also induced MDA5 and other MDA5 signaling components suggesting that positive feedback contributes to t1IFN dependent expression of the pro-inflammatory signature. Vascular permeability, induced by pICLC or another compound, inhibited Cg by limiting iron. These data suggest that pICLC induces t1IFN which potentiates pICLC-MDA5 signaling increasing IL-1 and IL-6 resulting in leakage of antimicrobial serum factors into lung airspace. Thus, induced vascular permeability may act as an innate defense mechanism against opportunistic fungal infection, such as cryptococcosis, and may be exploited as a host-directed therapeutic target.
Keywords: Cryptococcus; IL-1; edema; inflammation; interferon; lungs.
Copyright © 2022 Davis, Martin, Pinheiro, Hoke, Moyer, Mayer-Barber, Chang and Kwon-Chung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Pulmonary Iron Limitation Induced by Exogenous Type I IFN Protects Mice from Cryptococcus gattii Independently of T Cells.mBio. 2019 Jun 18;10(3):e00799-19. doi: 10.1128/mBio.00799-19. mBio. 2019. PMID: 31213551 Free PMC article.
-
Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis.PLoS Pathog. 2015 Aug 7;11(8):e1005040. doi: 10.1371/journal.ppat.1005040. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26252005 Free PMC article.
-
Exogenous Stimulation of Type I Interferon Protects Mice with Chronic Granulomatous Disease from Aspergillosis through Early Recruitment of Host-Protective Neutrophils into the Lung.mBio. 2018 Mar 27;9(2):e00422-18. doi: 10.1128/mBio.00422-18. mBio. 2018. PMID: 29588403 Free PMC article.
-
Cryptococcus neoformans Infection in Mice Lacking Type I Interferon Signaling Leads to Increased Fungal Clearance and IL-4-Dependent Mucin Production in the Lungs.PLoS One. 2015 Sep 18;10(9):e0138291. doi: 10.1371/journal.pone.0138291. eCollection 2015. PLoS One. 2015. PMID: 26384031 Free PMC article.
-
Type 1 Interferon in the Human Intestine-A Co-ordinator of the Immune Response to the Microbiota.Inflamm Bowel Dis. 2017 Apr;23(4):524-533. doi: 10.1097/MIB.0000000000001078. Inflamm Bowel Dis. 2017. PMID: 28296819 Review.
References
-
- Centers for Disease C.Prevention . Emergence of Cryptococcus gattii– pacific Northwest, 2004-2010. MMWR Morb Mortal Wkly Rep (2010) 59:865–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

