DNAM-1-chimeric receptor-engineered NK cells, combined with Nutlin-3a, more effectively fight neuroblastoma cells in vitro: a proof-of-concept study
- PMID: 35967339
- PMCID: PMC9367496
- DOI: 10.3389/fimmu.2022.886319
DNAM-1-chimeric receptor-engineered NK cells, combined with Nutlin-3a, more effectively fight neuroblastoma cells in vitro: a proof-of-concept study
Abstract
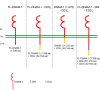
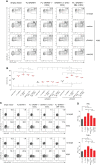
Adoptive transfer of engineered NK cells, one of clinical approaches to fight cancer, is gaining great interest in the last decade. However, the development of new strategies is needed to improve clinical efficacy and safety of NK cell-based immunotherapy. NK cell-mediated recognition and lysis of tumor cells are strictly dependent on the expression of ligands for NK cell-activating receptors NKG2D and DNAM-1 on tumor cells. Of note, the PVR/CD155 and Nectin-2/CD112 ligands for DNAM-1 are expressed primarily on solid tumor cells and poorly expressed in normal tissue cells. Here, we generated human NK cells expressing either the full length DNAM-1 receptor or three different DNAM-1-based chimeric receptor that provide the expression of DNAM-1 fused to a costimulatory molecule such as 2B4 and CD3ζ chain. Upon transfection into primary human NK cells isolated from healthy donors, we evaluated the surface expression of DNAM-1 and, as a functional readout, we assessed the extent of degranulation, cytotoxicity and the production of IFNγ and TNFα in response to human leukemic K562 cell line. In addition, we explored the effect of Nutlin-3a, a MDM2-targeting drug able of restoring p53 functions and known to have an immunomodulatory effect, on the degranulation of DNAM-1-engineered NK cells in response to human neuroblastoma (NB) LA-N-5 and SMS-KCNR cell lines. By comparing NK cells transfected with four different plasmid vectors and through blocking experiments, DNAM-1-CD3ζ-engineered NK cells showed the strongest response. Furthermore, both LA-N-5 and SMS-KCNR cells pretreated with Nutlin-3a were significantly more susceptible to DNAM-1-engineered NK cells than NK cells transfected with the empty vector. Our results provide a proof-of-concept suggesting that the combined use of DNAM-1-chimeric receptor-engineered NK cells and Nutlin-3a may represent a novel therapeutic approach for the treatment of solid tumors, such as NB, carrying dysfunctional p53.
Keywords: NK cells; activating receptor; adoptive transfer of NK and CAR-NK cells; chimeric receptor; immunomodulation; immunotherapy combined therapy.
Copyright © 2022 Focaccetti, Benvenuto, Pighi, Vitelli, Napolitano, Cotugno, Fruci, Palma, Rossi, Bei and Cifaldi.
Conflict of interest statement
Authors AV and FN are employed by ReiThera Srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Cifaldi L, Doria M, Cotugno N, Zicari S, Cancrini C, Palma P, et al. . DNAM-1 activating receptor and its ligands: How do viruses affect the NK cell-mediated immune surveillance during the various phases of infection? Int J Mol Sci (2019) 20(15), 3715, 1–21. doi: 10.3390/ijms20153715 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

