Six macrophage-associated genes in synovium constitute a novel diagnostic signature for osteoarthritis
- PMID: 35967352
- PMCID: PMC9368762
- DOI: 10.3389/fimmu.2022.936606
Six macrophage-associated genes in synovium constitute a novel diagnostic signature for osteoarthritis
Abstract
Background: Synovial macrophages play important roles in the formation and progression of osteoarthritis (OA). This study aimed to explore the biological and clinical significance of macrophage-associated genes (MAGs) in OA.
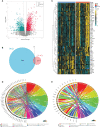
Methods: The OA synovial gene expression profiles GSE89408 and GSE82107 were obtained from the GEO database. Single-sample gene set enrichment analysis (ssGSEA) and GSEA were employed to decipher differences in immune infiltration and macrophage-associated biological pathways, respectively. Protein-protein interaction (PPI) network analysis and machine learning were utilized to establish a macrophage-associated gene diagnostic signature (MAGDS). RT-qPCR was performed to test the expression of key MAGs in murine models.
Results: OA synovium presented high levels of immune infiltration and activation of macrophage-associated biological pathways. A total of 55 differentially expressed MAGs were identified. Using PPI analysis and machine learning, a MAGDS consisting of IL1B, C5AR1, FCGR2B, IL10, IL6, and TYROBP was established for OA diagnosis (AUC = 0.910) and molecular pathological evaluation. Patients with high MAGDS scores may possess higher levels of immune infiltration and expression of matrix metalloproteinases (MMPs), implying poor biological alterations. The diagnostic value of MAGDS was also validated in an external cohort (AUC = 0.886). The expression of key MAGs was validated in a murine model using RT-qPCR. Additionally, a competitive endogenous RNA network was constructed to reveal the potential posttranscriptional regulatory mechanisms.
Conclusions: We developed and validated a MAGDS model with the ability to accurately diagnose and characterize biological alterations in OA. The six key MAGs may also be latent targets for immunoregulatory therapy.
Keywords: Osteoarthritis; diagnostic signature; immunopathology; machine learning; macrophage-associated genes; synovial macrophages.
Copyright © 2022 Liu, Lu, Liu, Ning, Li, Chen, Ge, Guo, Zheng, Wei and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

