Real-life data on monoclonal antibodies and antiviral drugs in Italian inborn errors of immunity patients during COVID-19 pandemic
- PMID: 35967382
- PMCID: PMC9367468
- DOI: 10.3389/fimmu.2022.947174
Real-life data on monoclonal antibodies and antiviral drugs in Italian inborn errors of immunity patients during COVID-19 pandemic
Abstract
Background: Since the beginning of the COVID-19 pandemic, patients with Inborn Errors of Immunity have been infected by SARS-CoV-2 virus showing a spectrum of disease ranging from asymptomatic to severe COVID-19. A fair number of patients did not respond adequately to SARS-CoV-2 vaccinations, thus early therapeutic or prophylactic measures were needed to prevent severe or fatal course or COVID-19 and to reduce the burden of hospitalizations.
Methods: Longitudinal, multicentric study on patients with Inborn Errors of Immunity immunized with mRNA vaccines treated with monoclonal antibodies and/or antiviral agents at the first infection and at reinfection by SARS-CoV-2. Analyses of efficacy were performed according to the different circulating SARS-CoV-2 strains.
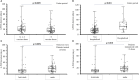
Results: The analysis of the cohort of 192 SARS-CoV-2 infected patients, across 26 months, showed the efficacy of antivirals on the risk of hospitalization, while mabs offered a positive effect on hospitalization, and COVID-19 severity. This protection was consistent across the alpha, delta and early omicron waves, although the emergence of BA.2 reduced the effect of available mabs. Hospitalized patients treated with mabs and antivirals had a lower risk of ICU admission. We reported 16 re-infections with a length of SARS-CoV-2 positivity at second infection shorter among patients treated with mabs. Treatment with antivirals and mabs was safe.
Conclusions: The widespread use of specific therapy, vaccination and better access to care might have contributed to mitigate risk of mortality, hospital admission, and severe disease. However, the rapid spread of new viral strains underlines that mabs and antiviral beneficial effects should be re- evaluated over time.
Keywords: COVID-19; antiviral drugs; disease severity; duration of viral shedding; hospitalization-risk; inborn errors of immunity; monoclonal antibodies; reinfection.
Copyright © 2022 Garzi, Cinetto, Firinu, Di Napoli, Lagnese, Punziano, Bez, Cinicola, Costanzo, Scarpa, Pulvirenti, Rattazzi, Spadaro, Quinti and Milito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Exploring the Use of Monoclonal Antibodies and Antiviral Therapies for Early Treatment of COVID-19 Outpatients in a Real-World Setting: A Nationwide Study from England and Italy.BioDrugs. 2023 Sep;37(5):675-684. doi: 10.1007/s40259-023-00601-w. Epub 2023 May 6. BioDrugs. 2023. PMID: 37148526 Free PMC article.
-
The evaluation of risk factors for prolonged viral shedding during anti-SARS-CoV-2 monoclonal antibodies and long-term administration of antivirals in COVID-19 patients with B-cell lymphoma treated by anti-CD20 antibody.BMC Infect Dis. 2024 Jul 22;24(1):715. doi: 10.1186/s12879-024-09631-3. BMC Infect Dis. 2024. PMID: 39039440 Free PMC article.
-
Early administration of nirmatrelvir/ritonavir leads to faster negative SARS-CoV-2 nasal swabs than monoclonal antibodies in COVID 19 patients at high-risk for severe disease.Virol J. 2024 Mar 20;21(1):68. doi: 10.1186/s12985-024-02333-x. Virol J. 2024. PMID: 38509536 Free PMC article.
-
Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis.Microbiol Spectr. 2022 Aug 31;10(4):e0092622. doi: 10.1128/spectrum.00926-22. Epub 2022 Jun 14. Microbiol Spectr. 2022. PMID: 35700134 Free PMC article.
-
Advances in the Omicron variant development.J Intern Med. 2022 Jul;292(1):81-90. doi: 10.1111/joim.13478. Epub 2022 Mar 22. J Intern Med. 2022. PMID: 35289434 Free PMC article. Review.
Cited by
-
A beacon in the dark: COVID-19 course in CVID patients from two European countries: Different approaches, similar outcomes.Front Immunol. 2023 Feb 8;14:1093385. doi: 10.3389/fimmu.2023.1093385. eCollection 2023. Front Immunol. 2023. PMID: 36845159 Free PMC article.
-
Early Treatment with Monoclonal Antibodies or Convalescent Plasma Reduces Mortality in Non-Vaccinated COVID-19 High-Risk Patients.Viruses. 2022 Dec 30;15(1):119. doi: 10.3390/v15010119. Viruses. 2022. PMID: 36680159 Free PMC article.
-
Lessons learned and implications of early therapies for coronavirus disease in a territorial service centre in the Calabria region: a retrospective study.BMC Infect Dis. 2022 Oct 20;22(1):793. doi: 10.1186/s12879-022-07774-9. BMC Infect Dis. 2022. PMID: 36266619 Free PMC article.
-
SARS-CoV-2 pre-exposure prophylaxis with tixagevimab/cilgavimab (AZD7442) provides protection in inborn errors of immunity with antibody defects: a real-world experience.Front Immunol. 2023 Oct 26;14:1249462. doi: 10.3389/fimmu.2023.1249462. eCollection 2023. Front Immunol. 2023. PMID: 37954618 Free PMC article.
-
Case report: Sotrovimab, remdesivir and nirmatrelvir/ritonavir combination as salvage treatment option in two immunocompromised patients hospitalized for COVID-19.Front Med (Lausanne). 2023 Jan 9;9:1062450. doi: 10.3389/fmed.2022.1062450. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36698815 Free PMC article.
References
-
- Available at: https://covid19.who.int/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

