Impaired sweating in patients with cholinergic urticaria is linked to low expression of acetylcholine receptor CHRM3 and acetylcholine esterase in sweat glands
- PMID: 35967390
- PMCID: PMC9373796
- DOI: 10.3389/fimmu.2022.955161
Impaired sweating in patients with cholinergic urticaria is linked to low expression of acetylcholine receptor CHRM3 and acetylcholine esterase in sweat glands
Abstract
Background: Cholinergic urticaria (CholU), a frequent form of chronic inducible urticaria, is characterized by itchy wheals and angioedema in response to sweating. As of now, the rate and pathophysiological relevance of impaired sweating in patients with CholU are ill-defined.
Aim: To assess in CholU patients the rate and extent of impaired sweating and its links to clinical and pathophysiological features of CholU.
Patients and methods: We assessed sweating in patients with CholU (n = 13) subjected to pulse-controlled ergometry (PCE) provocation testing. Pre- and post-PCE biopsies of lesional (L) and non-lesional (NL) skin were analyzed for the expression of acetylcholine receptor M3 (CHRM3) and acetylcholine esterase (ACh-E) by quantitative histomorphometry and compared to those of healthy control subjects (HCs). CholU patients were assessed for disease duration and severity as well as other clinical features.
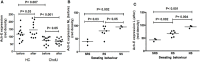
Results: Of the 13 patients with CholU, 10 showed reduced sweating in response to PCE provocation, and 3 had severely reduced sweating. Reduced sweating was linked to long disease duration and high disease severity. CholU patients with impaired sweating responses showed reduced sweat gland epithelial expression of CHRM3 and ACh-E.
Conclusion: Reduced sweating is common in CholU patients, especially in those with long-standing and severe disease, and it can be severe. Reduced expression of CHRM3 and ACh-E may be the cause or consequence of CholU in patients with impaired sweating, and this should be explored by further studies.
Keywords: acetylcholine esterase; cholinergic; hypohidrosis/anhidrosis; muscarinic 3 receptor; sweat gland; urticaria; wheal.
Copyright © 2022 Wang, Scheffel, Vera, Liu, Günzel, Terhorst-Molawi, Maurer and Altrichter.
Conflict of interest statement
DT-M has received research funds/was an advisor for Celldex, Novartis, Sanofi, and Moxie. MM is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third HarmonicBio, UCB, and Uriach. SA has been a speaker and/or advisor for and/or has conducted studies for AstraZeneca, Allakos, GSK, Leo Pharma, Lilly, Moxie, Novartis, Thermo Fisher, and Sanofi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Altered Sweat Composition Due to Changes in Tight Junction Expression of Sweat Glands in Cholinergic Urticaria Patients.Int J Mol Sci. 2024 Apr 25;25(9):4658. doi: 10.3390/ijms25094658. Int J Mol Sci. 2024. PMID: 38731882 Free PMC article.
-
New Etiology of Cholinergic Urticaria.Curr Probl Dermatol. 2016;51:94-100. doi: 10.1159/000446787. Epub 2016 Aug 30. Curr Probl Dermatol. 2016. PMID: 27584968 Review.
-
Acetylcholine-induced whealing in cholinergic urticaria - What does it tell us?J Dermatol Sci. 2021 Jul;103(1):10-15. doi: 10.1016/j.jdermsci.2021.05.001. Epub 2021 May 7. J Dermatol Sci. 2021. PMID: 34049770 Clinical Trial.
-
Direct and indirect action modes of acetylcholine in cholinergic urticaria.Allergol Int. 2021 Jan;70(1):39-44. doi: 10.1016/j.alit.2020.05.006. Epub 2020 Jun 19. Allergol Int. 2021. PMID: 32565175 Review.
-
Cholinergic Urticaria: Subtype Classification and Clinical Approach.Am J Clin Dermatol. 2023 Jan;24(1):41-54. doi: 10.1007/s40257-022-00728-6. Epub 2022 Sep 15. Am J Clin Dermatol. 2023. PMID: 36107396 Free PMC article. Review.
Cited by
-
Cross-sectional study of cholinergic urticaria subtypes and bronchial hyperresponsiveness.Sci Rep. 2022 Oct 27;12(1):18122. doi: 10.1038/s41598-022-22655-6. Sci Rep. 2022. PMID: 36302805 Free PMC article.
-
Altered Sweat Composition Due to Changes in Tight Junction Expression of Sweat Glands in Cholinergic Urticaria Patients.Int J Mol Sci. 2024 Apr 25;25(9):4658. doi: 10.3390/ijms25094658. Int J Mol Sci. 2024. PMID: 38731882 Free PMC article.
-
New insights into chronic inducible urticaria.Curr Allergy Asthma Rep. 2024 Aug;24(8):457-469. doi: 10.1007/s11882-024-01160-y. Epub 2024 Jul 19. Curr Allergy Asthma Rep. 2024. PMID: 39028396 Free PMC article. Review.
-
Decoding Autoimmune Autonomic Disorders: A Less-Recognized Overlap.Ann Indian Acad Neurol. 2024 Sep 1;27(5):482-492. doi: 10.4103/aian.aian_394_24. Epub 2024 Oct 8. Ann Indian Acad Neurol. 2024. PMID: 39377234 Free PMC article.
-
Research progress in the pathogenesis of chronic urticaria.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Oct 28;48(10):1602-1610. doi: 10.11817/j.issn.1672-7347.2023.230037. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 38432889 Free PMC article. Chinese, English.
References
-
- Magerl M, Altrichter S, Borzova E, Giménez-Arnau A, Grattan CE, Lawlor F, et al. . The definition, diagnostic testing, and management of chronic inducible urticarias - the EAACI/GA(2) LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy (2016) 71(6):780–802. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

