A recombinant murine-like rotavirus with Nano-Luciferase expression reveals tissue tropism, replication dynamics, and virus transmission
- PMID: 35967392
- PMCID: PMC9372724
- DOI: 10.3389/fimmu.2022.911024
A recombinant murine-like rotavirus with Nano-Luciferase expression reveals tissue tropism, replication dynamics, and virus transmission
Abstract
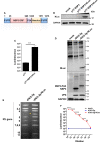
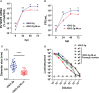
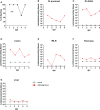
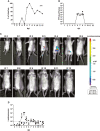
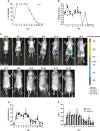
Rotaviruses (RVs) are one of the main causes of severe gastroenteritis, diarrhea, and death in children and young animals. While suckling mice prove to be highly useful small animal models of RV infection and pathogenesis, direct visualization tools are lacking to track the temporal dynamics of RV replication and transmissibility in vivo. Here, we report the generation of the first recombinant murine-like RV that encodes a Nano-Luciferase reporter (NLuc) using a newly optimized RV reverse genetics system. The NLuc-expressing RV was replication-competent in cell culture and both infectious and virulent in neonatal mice in vivo. Strong luciferase signals were detected in the proximal and distal small intestines, colon, and mesenteric lymph nodes. We showed, via a noninvasive in vivo imaging system, that RV intestinal replication peaked at days 2 to 5 post infection. Moreover, we successfully tracked RV transmission to uninoculated littermates as early as 3 days post infection, 1 day prior to clinically apparent diarrhea and 3 days prior to detectable fecal RV shedding in the uninoculated littermates. We also observed significantly increased viral replication in Stat1 knockout mice that lack the host interferon signaling. Our results suggest that the NLuc murine-like RV represents a non-lethal powerful tool for the studies of tissue tropism and host and viral factors that regulate RV replication and spread, as well as provides a new tool to facilitate the testing of prophylactic and therapeutic interventions in the future.
Keywords: Nano-luciferase; in vivo imaging system; rotavirus; tissue tropism; transmission.
Copyright © 2022 Zhu, Sánchez-Tacuba, Hou, Kawagishi, Feng, Greenberg and Ding.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Reverse Genetics of Murine Rotavirus: A Comparative Analysis of the Wild-Type and Cell-Culture-Adapted Murine Rotavirus VP4 in Replication and Virulence in Neonatal Mice.Viruses. 2024 May 12;16(5):767. doi: 10.3390/v16050767. Viruses. 2024. PMID: 38793648 Free PMC article.
-
Rotavirus NSP1 Contributes to Intestinal Viral Replication, Pathogenesis, and Transmission.mBio. 2021 Dec 21;12(6):e0320821. doi: 10.1128/mBio.03208-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903043 Free PMC article.
-
The Role of the VP4 Attachment Protein in Rotavirus Host Range Restriction in an In Vivo Suckling Mouse Model.J Virol. 2022 Aug 10;96(15):e0055022. doi: 10.1128/jvi.00550-22. Epub 2022 Jul 12. J Virol. 2022. PMID: 35862708 Free PMC article.
-
Rotaviruses and rotavirus vaccines.Br Med Bull. 2009;90:37-51. doi: 10.1093/bmb/ldp009. Epub 2009 Feb 20. Br Med Bull. 2009. PMID: 19233929 Review.
-
Rotavirus reverse genetics: A tool for understanding virus biology.Virus Res. 2021 Nov;305:198576. doi: 10.1016/j.virusres.2021.198576. Epub 2021 Sep 21. Virus Res. 2021. PMID: 34560180 Review.
Cited by
-
Rhesus rotavirus NSP1 mediates extra-intestinal infection and is a contributing factor for biliary obstruction.PLoS Pathog. 2024 Sep 30;20(9):e1012609. doi: 10.1371/journal.ppat.1012609. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39348381 Free PMC article.
-
Cryptosporidium infection of human small intestinal epithelial cells induces type III interferon and impairs infectivity of Rotavirus.Gut Microbes. 2024 Jan-Dec;16(1):2297897. doi: 10.1080/19490976.2023.2297897. Epub 2024 Jan 8. Gut Microbes. 2024. PMID: 38189373 Free PMC article.
-
CRISPR/Cas9 screens identify key host factors that enhance rotavirus reverse genetics efficacy and vaccine production.NPJ Vaccines. 2024 Nov 6;9(1):211. doi: 10.1038/s41541-024-01007-7. NPJ Vaccines. 2024. PMID: 39505878 Free PMC article.
-
Reverse Genetics of Murine Rotavirus: A Comparative Analysis of the Wild-Type and Cell-Culture-Adapted Murine Rotavirus VP4 in Replication and Virulence in Neonatal Mice.Viruses. 2024 May 12;16(5):767. doi: 10.3390/v16050767. Viruses. 2024. PMID: 38793648 Free PMC article.
-
Re-Examining Rotavirus Innate Immune Evasion: Potential Applications of the Reverse Genetics System.mBio. 2022 Aug 30;13(4):e0130822. doi: 10.1128/mbio.01308-22. Epub 2022 Jun 14. mBio. 2022. PMID: 35699371 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

