Case Report: Successful treatment of late-onset immune checkpoint inhibitor-associated membranous nephropathy in a patient with advanced renal cell carcinoma
- PMID: 35967405
- PMCID: PMC9366044
- DOI: 10.3389/fimmu.2022.898811
Case Report: Successful treatment of late-onset immune checkpoint inhibitor-associated membranous nephropathy in a patient with advanced renal cell carcinoma
Abstract
Background: Diagnosing immune checkpoint inhibitor (ICI)-associated nephritis can be challenging since it is a rare complication of therapy, associated with a spectrum of immune-mediated pathologies, and can present months after ICI therapy discontinuation (i.e., late-onset). ICIs are increasingly administered in combination with other cancer therapies with associated nephrotoxicity, further obfuscating the diagnosis of ICI-associated nephritis. In this report, we describe the first suspected case of late-onset ICI-associated membranous nephropathy (MN) in a patient with metastatic clear cell renal cell carcinoma (RCC) who had discontinued ICI therapy 6 months prior to presentation. Prompt recognition of the suspected late-onset immune-related adverse event (irAE) resulted in the successful treatment of MN and continuation of RCC therapy.
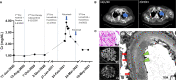
Case presentation: A 57-year-old man with metastatic clear cell RCC was responsive to third-line RCC therapy with lenvatinib (oral TKI) and everolimus (oral mTOR inhibitor) when he presented with nephrotic range proteinuria and acute kidney injury (AKI). His kidney biopsy revealed probable secondary MN with subendothelial and mesangial immune complex deposits and negative staining for both phospholipase A2 receptor (PLA2R) and thrombospondin type-1 domain-containing 7A (THSD7A). While a diagnosis of paraneoplastic MN could not be excluded, the patient was responding to cancer therapy and had tumor regression. However, 6 months prior to presentation, the patient had received pembrolizumab, an ICI, with his first-line RCC treatment. Due to concern that the patient may be presenting with late-onset ICI-associated MN, he was effectively treated with rituximab, which allowed for his continued RCC therapy.
Conclusion: This report highlights the first case of suspected late-onset ICI-associated MN and the increasing complexity of recognizing renal irAEs. With the growing indications for the use of ICIs in combination with other cancer therapies, recognizing the various presentations of ICI-immune nephritis can help guide patient management and treatment.
Keywords: case report; immune checkpoint inhibitor (ICI); immune related adverse events (irAEs); membranous nephropathy (MN); proteinuria; renal cell carcinoma (RCC); rituximab.
Copyright © 2022 Ratanasrimetha, Reddy, Kala, Tchakarov, Glass, Msaouel and Lin.
Conflict of interest statement
PM has received honoraria for service on a Scientific Advisory Board for Mirati Therapeutics, Bristol Myers Squibb, and Exelixis; consulting for Axiom Healthcare Strategies; non-branded educational programs supported by Exelixis and Pfizer; and research funding for clinical trials from Takeda, Bristol Myers Squibb, Mirati Therapeutics, Gateway for Cancer Research, and UT MD Anderson Cancer Center. JK is on the speaker panel for BTG International. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

