Spindle pole body component 25 and platelet-derived growth factor mediate crosstalk between tumor-associated macrophages and prostate cancer cells
- PMID: 35967419
- PMCID: PMC9363606
- DOI: 10.3389/fimmu.2022.907636
Spindle pole body component 25 and platelet-derived growth factor mediate crosstalk between tumor-associated macrophages and prostate cancer cells
Abstract
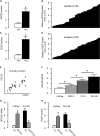
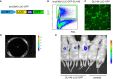
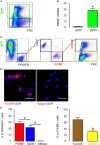
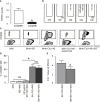
Tumor-associated macrophages (TAMs) are involved in the growth of prostate cancer (PrC), while the molecular mechanisms underlying the interactive crosstalk between TAM and PrC cells remain largely unknown. Platelet-derived growth factor (PDGF) is known to promote mesenchymal stromal cell chemotaxis to the tumor microenvironment. Recently, activation of spindle pole body component 25 (SPC25) has been shown to promote PrC cell proliferation and is associated with PrC stemness. Here, the relationship between SPC25 and PDGF in the crosstalk between TAM and PrC was investigated. Significant increases in both PDGF and SPC25 levels were detected in PrC specimens compared to paired adjacent normal prostate tissues. A significant correlation was detected between PDGF and SPC25 levels in PrC specimens and cell lines. SPC25 increased PDGF production and tumor cell growth in cultured PrC cells and in xenotransplantation. Mechanistically, SPC25 appeared to activate PDGF in PrC likely through Early Growth Response 1 (Egr1), while the secreted PDGF signaled to TAM through PDGFR on macrophages and polarized macrophages, which, in turn, induced the growth of PrC cells likely through their production and secretion of transforming growth factor β1 (TGFβ1). Thus, our data suggest that SPC25 triggers the crosstalk between TAM and PrC cells via SPC25/PDGF/PDGFR/TGFβ1 receptor signaling to enhance PrC growth.
Keywords: SPC25; crosstalk; pdgf; prostate cancer; tumor associated macrophage (TAM).
Copyright © 2022 Cui, Xu, Hu and Lv.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Spindle pole body component 25 regulates stemness of prostate cancer cells.Aging (Albany NY). 2018 Nov 8;10(11):3273-3282. doi: 10.18632/aging.101631. Aging (Albany NY). 2018. PMID: 30408771 Free PMC article.
-
SPC25 Functions as a Prognostic-Related Biomarker, and Its High Expression Correlates with Tumor Immune Infiltration and UCEC Progression.Front Biosci (Landmark Ed). 2024 Feb 20;29(2):69. doi: 10.31083/j.fbl2902069. Front Biosci (Landmark Ed). 2024. PMID: 38420826
-
Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases.J Natl Cancer Inst. 2003 Mar 19;95(6):458-70. doi: 10.1093/jnci/95.6.458. J Natl Cancer Inst. 2003. PMID: 12644539
-
New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies.Biomed Pharmacother. 2023 May;161:114491. doi: 10.1016/j.biopha.2023.114491. Epub 2023 Mar 13. Biomed Pharmacother. 2023. PMID: 37002577 Review.
-
PDGF/PDGFR signaling and targeting in cancer growth and progression: Focus on tumor microenvironment and cancer-associated fibroblasts.Curr Pharm Des. 2014;20(17):2843-8. doi: 10.2174/13816128113199990592. Curr Pharm Des. 2014. PMID: 23944365 Review.
Cited by
-
The role of angiogenic growth factors in the immune microenvironment of glioma.Front Oncol. 2023 Sep 13;13:1254694. doi: 10.3389/fonc.2023.1254694. eCollection 2023. Front Oncol. 2023. PMID: 37790751 Free PMC article. Review.
-
Omics Integration Uncovers Mechanisms Associated with HIV Viral Load and Potential Therapeutic Insights.medRxiv [Preprint]. 2025 Jul 30:2025.07.29.25332397. doi: 10.1101/2025.07.29.25332397. medRxiv. 2025. PMID: 40766151 Free PMC article. Preprint.
-
Mefloquine Suppresses Metastasis in Renal Cell Carcinoma Through Targeting SPC25.Cancer Sci. 2025 May;116(5):1239-1254. doi: 10.1111/cas.70001. Epub 2025 Feb 13. Cancer Sci. 2025. PMID: 39948743 Free PMC article.
-
SPC25 as a novel therapeutic and prognostic biomarker and its association with glycolysis, ferroptosis and ceRNA in lung adenocarcinoma.Aging (Albany NY). 2024 Jan 11;16(1):779-798. doi: 10.18632/aging.205418. Epub 2024 Jan 11. Aging (Albany NY). 2024. PMID: 38217547 Free PMC article.
-
Cancer stem cells: advances in knowledge and implications for cancer therapy.Signal Transduct Target Ther. 2024 Jul 5;9(1):170. doi: 10.1038/s41392-024-01851-y. Signal Transduct Target Ther. 2024. PMID: 38965243 Free PMC article. Review.
References
-
- Fonseka LN, Kallen ME, Serrato-Guillen A, Chow R, Tirado CA. Cytogenetics and molecular genetics of prostate cancer: a comprehensive update. J Assoc Genet Technol (2015) 41:100–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

