TCR-like antibodies targeting autoantigen-mhc complexes: a mini-review
- PMID: 35967436
- PMCID: PMC9363607
- DOI: 10.3389/fimmu.2022.968432
TCR-like antibodies targeting autoantigen-mhc complexes: a mini-review
Abstract
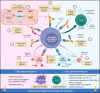
T cell receptors (TCRs) recognize peptide antigens bound to major histocompatibility complex (MHC) molecules (p/MHC) that are expressed on cell surfaces; while B cell-derived antibodies (Abs) recognize soluble or cell surface native antigens of various types (proteins, carbohydrates, etc.). Immune surveillance by T and B cells thus inspects almost all formats of antigens to mount adaptive immune responses against cancer cells, infectious organisms and other foreign insults, while maintaining tolerance to self-tissues. With contributions from environmental triggers, the development of autoimmune disease is thought to be due to the expression of MHC risk alleles by antigen-presenting cells (APCs) presenting self-antigen (autoantigen), breaking through self-tolerance and activating autoreactive T cells, which orchestrate downstream pathologic events. Investigating and treating autoimmune diseases have been challenging, both because of the intrinsic complexity of these diseases and the need for tools targeting T cell epitopes (autoantigen-MHC). Naturally occurring TCRs with relatively low (micromolar) affinities to p/MHC are suboptimal for autoantigen-MHC targeting, whereas the use of engineered TCRs and their derivatives (e.g., TCR multimers and TCR-engineered T cells) are limited by unpredictable cross-reactivity. As Abs generally have nanomolar affinity, recent advances in engineering TCR-like (TCRL) Abs promise advantages over their TCR counterparts for autoantigen-MHC targeting. Here, we compare the p/MHC binding by TCRs and TCRL Abs, review the strategies for generation of TCRL Abs, highlight their application for identification of autoantigen-presenting APCs, and discuss future directions and limitations of TCRL Abs as immunotherapy for autoimmune diseases.
Keywords: TCR-like antibodies; antigen-specific therapy; autoantigen presentation; autoimmune diseases; immunotherapy.
Copyright © 2022 Li, Jiang and Mellins.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Harnessing antibody-mediated recognition of the intracellular proteome with T cell receptor-like specificity.Front Immunol. 2024 Nov 22;15:1486721. doi: 10.3389/fimmu.2024.1486721. eCollection 2024. Front Immunol. 2024. PMID: 39650646 Free PMC article. Review.
-
Antigen-specific modulation of chronic experimental autoimmune encephalomyelitis in humanized mice by TCR-like antibody targeting autoreactive T-cell epitope.Life Sci Alliance. 2024 Nov 4;8(1):e202402996. doi: 10.26508/lsa.202402996. Print 2025 Jan. Life Sci Alliance. 2024. PMID: 39496501 Free PMC article.
-
Recombinant antibodies with MHC-restricted, peptide-specific, T-cell receptor-like specificity: new tools to study antigen presentation and TCR-peptide-MHC interactions.J Mol Recognit. 2003 Sep-Oct;16(5):324-32. doi: 10.1002/jmr.640. J Mol Recognit. 2003. PMID: 14523945 Review.
-
Antigen-specific immunomodulation for type 1 diabetes by novel recombinant antibodies directed against diabetes-associates auto-reactive T cell epitope.J Autoimmun. 2013 Dec;47:83-93. doi: 10.1016/j.jaut.2013.08.009. Epub 2013 Oct 3. J Autoimmun. 2013. PMID: 24090977
-
Autoimmune diabetes: the role of T cells, MHC molecules and autoantigens.Autoimmunity. 1998;27(3):159-77. doi: 10.3109/08916939809003864. Autoimmunity. 1998. PMID: 9609134 Review.
Cited by
-
Exploring the research progression and evolutionary trends of gut microbiome and hypertension: a bibliometric analysis.Front Microbiol. 2025 May 20;16:1530857. doi: 10.3389/fmicb.2025.1530857. eCollection 2025. Front Microbiol. 2025. PMID: 40463443 Free PMC article.
-
The Future of TCR-like Antibodies in Diagnosis and Potential Application Targets.Curr Mol Med. 2025;25(6):672-685. doi: 10.2174/0115665240297179240514030532. Curr Mol Med. 2025. PMID: 38803176 Review.
-
Inflammatory Response: A Crucial Way for Gut Microbes to Regulate Cardiovascular Diseases.Nutrients. 2023 Jan 24;15(3):607. doi: 10.3390/nu15030607. Nutrients. 2023. PMID: 36771313 Free PMC article. Review.
-
A general platform for targeting MHC-II antigens via a single loop.bioRxiv [Preprint]. 2024 Jan 30:2024.01.26.577489. doi: 10.1101/2024.01.26.577489. bioRxiv. 2024. PMID: 38352315 Free PMC article. Preprint.
-
Harnessing antibody-mediated recognition of the intracellular proteome with T cell receptor-like specificity.Front Immunol. 2024 Nov 22;15:1486721. doi: 10.3389/fimmu.2024.1486721. eCollection 2024. Front Immunol. 2024. PMID: 39650646 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

