The impact of antibiotic use on clinical features and survival outcomes of cancer patients treated with immune checkpoint inhibitors
- PMID: 35967438
- PMCID: PMC9367677
- DOI: 10.3389/fimmu.2022.968729
The impact of antibiotic use on clinical features and survival outcomes of cancer patients treated with immune checkpoint inhibitors
Abstract
Background: Nowadays, immune checkpoint inhibitors (ICIs) have become one of the essential immunotherapies for cancer patients. However, the impact of antibiotic (ATB) use on cancer patients treated with ICIs remains controversial.
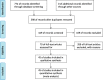
Methods: Our research included retrospective studies and a randomized clinical trial (RCT) with cancer patients treated with ICIs and ATB, from the public database of PubMed, Web of Science, Embase, Cochrane, clinical trials, and JAMA. The survival outcomes included progression-free survival (PFS) and overall survival (OS). Meanwhile, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, and subgroup analyses were performed to determine the concrete association between ATB use and the prognosis of cancer patients treated in ICIs.
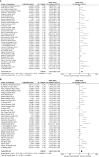
Results: Our results revealed that ATB use was associated with poor survival outcomes, including OS (HR: 1.94, 95% CI: 1.68-2.25, p <0.001) and PFS (HR: 1.83, 95% CI: 1.53-2.19, p <0.001). The subgroup analysis learned about the association between ATB use and the prognosis of cancer patients with ICI treatment, including 5 cancer types, 3 kinds of ICI, 5 different ATP windows, broad-spectrum ATB class, and ECOG score. ATB treatment was associated with poor OS of non-small-cell lung cancer (NSCLC), renal cell carcinoma (RCC), esophageal cancer (EC), and melanoma (MEL) in patients treated in ICIs, while non-small-cell lung cancer (NSCLC) and renal cell carcinoma (RCC) were associated with poor PFS. Meanwhile, it was strongly related to the ICI type and ATB window. Furthermore, it is firstly mentioned that the use of broad-spectrum ATB class was strongly associated with poor PFS.
Conclusion: In conclusion, our meta-analysis indicated that ATB use was significantly associated with poor OS and PFS of cancer patients treated with ICI immunotherapy, especially for patients with ATB use in the period of (-60 days; +30 days) near the initiation of ICI treatment. Also, different cancer types and the ICI type can also impact the survival outcome. This first reveals the strong relationship between the broad-spectrum ATB class and poor PFS. Still, more studies are needed for further study.
Keywords: PD-1; PD-L1; antibiotic; immune checkpoint inhibitor; survival outcomes.
Copyright © 2022 Zhou, Huang, Wong, Hu, Zhu, Li and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





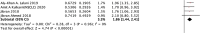
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

