Aedes aegypti CLIPB9 activates prophenoloxidase-3 in the presence of CLIPA14 after fungal infection
- PMID: 35967454
- PMCID: PMC9365933
- DOI: 10.3389/fimmu.2022.927322
Aedes aegypti CLIPB9 activates prophenoloxidase-3 in the presence of CLIPA14 after fungal infection
Abstract
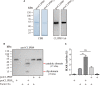
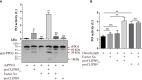
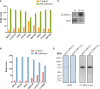
Melanization is an integral part of the insect defense system and is often induced by pathogen invasion. Phenoloxidases (POs) are critical enzymes that catalyze melanin formation. PO3 is associated with the antifungal response of the mosquito, Aedes aegypti, but the molecular mechanism of the prophenoloxidase-3 (PPO3) activation is unclear. Here we report that PPO3 cleavage activation is mediated by a clip-domain serine protease, CLIPB9. We purified recombinant CLIPB9 and found that it cleaved PPO3 and increased PO activity in the hemolymph. We then identified CLIPA14 (a serine protease homolog) by co-immunoprecipitation using anti-CLIPB9 antibody. After being cleaved by CLIPB9, Ae. aegypti CLIPA14 acted as a cofactor for PPO3 activation. In addition, dsRNA co-silencing of CLIPB9 and CLIPA14 genes reduced melanization after infection with the entomopathogen, Beauveria bassiana, making the adult mosquitoes more sensitive to fungal infection. These results illustrate the roles of CLIPB9 and CLIPA14 in the PPO activation pathway and revealed the complexity of the upstream serine protease network controlling melanization.
Keywords: antifungal response; immune melanization; prophenoloxidase; serine protease; serine protease homolog.
Copyright © 2022 Ji, Lu, Zou and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Activation of Aedes aegypti prophenoloxidase-3 and its role in the immune response against entomopathogenic fungi.Insect Mol Biol. 2017 Oct;26(5):552-563. doi: 10.1111/imb.12318. Epub 2017 May 27. Insect Mol Biol. 2017. PMID: 28556276 Free PMC article.
-
The serine protease homolog CLIPA14 modulates the intensity of the immune response in the mosquito Anopheles gambiae.J Biol Chem. 2017 Nov 3;292(44):18217-18226. doi: 10.1074/jbc.M117.797787. Epub 2017 Sep 19. J Biol Chem. 2017. PMID: 28928218 Free PMC article.
-
CLIPB8 is part of the prophenoloxidase activation system in Anopheles gambiae mosquitoes.Insect Biochem Mol Biol. 2016 Apr;71:106-15. doi: 10.1016/j.ibmb.2016.02.008. Epub 2016 Feb 27. Insect Biochem Mol Biol. 2016. PMID: 26926112 Free PMC article.
-
Regulation and function of the melanization reaction in Drosophila.Fly (Austin). 2009 Jan-Mar;3(1):105-11. doi: 10.4161/fly.3.1.7747. Epub 2009 Jan 2. Fly (Austin). 2009. PMID: 19164947 Review.
-
Serine proteases as mediators of mosquito immune responses.Insect Biochem Mol Biol. 2001 Mar 1;31(3):257-62. doi: 10.1016/s0965-1748(00)00145-4. Insect Biochem Mol Biol. 2001. PMID: 11167095 Review.
Cited by
-
CLIPB4 is a central node in the protease network that regulates humoral immunity in Anopheles gambiae mosquitoes.bioRxiv [Preprint]. 2023 Jul 16:2023.07.07.545904. doi: 10.1101/2023.07.07.545904. bioRxiv. 2023. Update in: J Innate Immun. 2023;15(1):680-696. doi: 10.1159/000533898. PMID: 37461554 Free PMC article. Updated. Preprint.
-
Insight into the structural hierarchy of the protease cascade that regulates the mosquito melanization response.Microbes Infect. 2024 Jan-Feb;26(1-2):105245. doi: 10.1016/j.micinf.2023.105245. Epub 2023 Nov 1. Microbes Infect. 2024. PMID: 37918462 Free PMC article.
-
Serine protease homolog pairs CLIPA4-A6, A4-A7Δ, and A4-A12 act as cofactors for proteolytic activation of prophenoloxidase-2 and -7 in Anopheles gambiae.Insect Biochem Mol Biol. 2024 Jan;164:104048. doi: 10.1016/j.ibmb.2023.104048. Epub 2023 Dec 5. Insect Biochem Mol Biol. 2024. PMID: 38056530 Free PMC article.
-
Hemolymph protease-17b activates proHP6 to stimulate melanization and Toll signaling in Manduca sexta.Insect Biochem Mol Biol. 2024 Nov;174:104193. doi: 10.1016/j.ibmb.2024.104193. Epub 2024 Oct 13. Insect Biochem Mol Biol. 2024. PMID: 39406299
-
CLIPB4 Is a Central Node in the Protease Network that Regulates Humoral Immunity in Anopheles gambiae Mosquitoes.J Innate Immun. 2023;15(1):680-696. doi: 10.1159/000533898. Epub 2023 Sep 13. J Innate Immun. 2023. PMID: 37703846 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

