A multicenter, randomized controlled, non-inferiority trial, comparing nasal continuous positive airway pressure with nasal intermittent positive pressure ventilation as primary support before minimally invasive surfactant administration for preterm infants with respiratory distress syndrome (the NIV-MISA-RDS trial): Study protocol
- PMID: 35967549
- PMCID: PMC9372355
- DOI: 10.3389/fped.2022.968462
A multicenter, randomized controlled, non-inferiority trial, comparing nasal continuous positive airway pressure with nasal intermittent positive pressure ventilation as primary support before minimally invasive surfactant administration for preterm infants with respiratory distress syndrome (the NIV-MISA-RDS trial): Study protocol
Abstract
Background: Non-invasive ventilation (NIV) treatment has been developed to minimize lung damage and to avoid invasive mechanical ventilation (IMV) in preterm infants, especially in those with a gestational age of <30 weeks. Our hypothesis is that for preterm infants <30 weeks with potential to develop respiratory distress syndrome (RDS), nasal continuous positive airway pressure (NCPAP) is non-inferior to the nasal intermittent positive pressure ventilation (NIPPV) as primary respiratory support before minimal invasive surfactant administration (MISA).
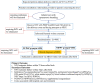
Methods and design: The NIV-MISA-RDS trial is planned as an unblinded, multicenter, randomized, non-inferiority trial at 14 tertiary neonatal intensive care units (NICUs) in China. Eligible infants are preterm infants of 24-29+6 weeks of gestational age who have spontaneous breaths at birth and require primary NIV support for RDS. Infants are randomized 1:1 to treatment with either NCPAP or NIPPV once admitted into NICUs. If an infant presents progressively aggravated respiratory distress and is clinically diagnosed as having RDS, pulmonary surfactant will be supplemented by MISA in the first 2 h of life. The primary outcome is NIV treatment failure within 72 h after birth. With a specified non-inferiority margin of 10%, using a two-sided 95% CI and 80% power, the study requires 480 infants per group (in total 960 infants).
Discussion: Current evidence shows that NIV and MISA may be the most effective strategy for minimizing IMV in preterm infants with RDS. However, there are few large randomized controlled trials to compare the effectiveness of NCPAP and NIPPV as the primary respiratory support after birth and before surfactant administration. We will conduct this trial to test the hypothesis that NCPAP is not inferior to NIPPV as the initial respiratory support in reducing the use of IMV in premature infants who have spontaneous breaths after birth and who do not require intubation in the first 2 h after birth. The study will provide clinical data for the selection of the initial non-invasive ventilation mode in preterm infants with a gestational age of <30 weeks with spontaneous breaths after birth.
Clinical trial registration: https://register.clinicaltrials.gov, identifier: NCT05137340.
Keywords: minimal invasive surfactant administration; nasal continuous positive airway pressure; nasal intermittent positive pressure ventilation; neonatal respiratory distress syndrome; preterm infants.
Copyright © 2022 Zhang, Li, Zeng, Gao, Zhao, Han and Tong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aziz K, Lee CHC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, et al. . Part 5: neonatal resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. (2021) 147 (Suppl. 1):e2020038505E. 10.1542/peds.2020-038505E - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

