Lactoferrin and Human Neutrophil Protein (HNP) 1-3 Levels During the Neonatal Period in Preterm Infants
- PMID: 35967550
- PMCID: PMC9364083
- DOI: 10.3389/fped.2022.909176
Lactoferrin and Human Neutrophil Protein (HNP) 1-3 Levels During the Neonatal Period in Preterm Infants
Abstract
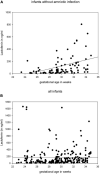
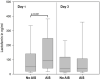
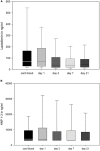
Antimicrobial polypeptides (APPs) are part of the innate immune system, but their specific role in the context of preterm birth is not yet understood. The aim of this investigation was to determine the systemic expression of APPs, i.e., lactoferrin (LF) and human neutrophil protein (HNP) 1-3 in preterm infants in the period of highest vulnerability for infection and to correlate these biomarkers with short-term outcome. We therefore conducted a prospective two-center study including plasma samples of 278 preterm infants and 78 corresponding mothers. APP levels were analyzed on day 1, 3, 7, and 21 of life via enzyme-linked immunosorbent assay (ELISA). The levels of LF and HNP1-3 remained stable during the first 21 days of life and were not influenced by maternal levels. Elevated APP levels were found at day 1 in infants born to mothers with amniotic infection syndrome (AIS vs. no AIS, mean ± SD in ng/ml: LF 199.8 ± 300 vs. 124.1 ± 216.8, HNP 1-3 16,819 ± 36,124 vs. 8,701 ± 11,840; p = 0.021, n = 179). We found no elevated levels of APPs before the onset of sepsis episodes or in association with other short-term outcomes that are in part mediated by inflammation such as necrotizing enterocolitis (NEC) or retinopathy of prematurity (ROP). Interestingly, infants developing bronchopulmonary dysplasia (BPD) showed higher levels of HNP1-3 on day 21 than infants without BPD (13,473 ± 16,135 vs. 8,388 ± 15,938, n = 111, p = 0.008). In infants born without amniotic infection, levels of the measured APPs correlated with gestational age and birth weight. In our longitudinal study, systemic levels of LF and HNP 1-3 were not associated with postnatal infection and adverse short-term outcomes in preterm infants.
Keywords: HNP 1–3; antimicrobial polypeptides; blood levels; infection; inflammation; lactoferrin; preterm infants.
Copyright © 2022 Faust, Moser, Bartels, Fortmann, Hanke, Wieg, Stichtenoth, Göpel, Herting and Härtel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Differential expression of antimicrobial polypeptides in cord blood samples of preterm and term infants.Acta Paediatr. 2014 Apr;103(4):e143-7. doi: 10.1111/apa.12544. Epub 2014 Jan 26. Acta Paediatr. 2014. PMID: 24387008
-
Is Mother's Own Milk Lactoferrin Intake Associated with Reduced Neonatal Sepsis, Necrotizing Enterocolitis, and Death?Neonatology. 2020;117(2):167-174. doi: 10.1159/000505663. Epub 2020 Feb 13. Neonatology. 2020. PMID: 32053823 Free PMC article.
-
Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells.Am J Perinatol. 2014 Dec;31(12):1111-20. doi: 10.1055/s-0034-1371704. Epub 2014 May 16. Am J Perinatol. 2014. PMID: 24839144 Clinical Trial.
-
Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia.Neonatology. 2015;107(4):358-9. doi: 10.1159/000381132. Epub 2015 Jun 5. Neonatology. 2015. PMID: 26044104 Review.
-
Enteral Lactoferrin Supplementation for Preventing Sepsis and Necrotizing Enterocolitis in Preterm Infants: A Meta‑Analysis With Trial Sequential Analysis of Randomized Controlled Trials.Front Pharmacol. 2020 Aug 7;11:1186. doi: 10.3389/fphar.2020.01186. eCollection 2020. Front Pharmacol. 2020. PMID: 32848789 Free PMC article. Review.
Cited by
-
The role of nutritional interventions in the prevention and treatment of chronic lung disease of prematurity.Pediatr Res. 2024 Apr 2. doi: 10.1038/s41390-024-03133-3. Online ahead of print. Pediatr Res. 2024. PMID: 38565917 Review.
References
-
- Wang HY, Chen XC, Yan ZH, Tu F, He T, Gopinath SCB, et al. Human neutrophil peptide 1 promotes immune sterilization in vivo by reducing the virulence of multidrug-resistant Klebsiella pneumoniae and increasing the ability of macrophages. Biotechnol Appl Biochem. (2021). 10.1002/bab.2270 [Epub ahead of print]. - DOI - PubMed
LinkOut - more resources
Full Text Sources

